Abstract
Purpose
Molecular data has become an essential part of the updated World Health Organization (WHO) grading of central nervous system tumors. However, stereotactic needle biopsies provide only small volume specimens and limit the extent of histologic and molecular testing that can be performed. We assessed the use of a tubular retractor-based minimally invasive biopsy technique to provide improved tissue yield and diagnostic data compared to needle biopsy.
Methods
Eighteen patients underwent an open transtubular biopsy compared to 146 stereotactic biopsies during the years of 2010–2018.
Results
Tubular biopsies resulted in a higher volume of tissue provided to the pathologist than needle biopsies (1.26 cm3 vs. 0.3 cm3; p < 0.0001). There was a higher rate of non-diagnostic sample with stereotactic compared to transtubular biopsy (13% vs. 0%; p = 0.13). Six patients who underwent stereotactic biopsy required reoperation for diagnosis, while no transtubular biopsy patient required reoperation in order to obtain a diagnostic specimen. Postoperative hematoma was the most common post-operative complication in both groups.
Conclusions
Stereotactic transtubular biopsies are a viable alternative to stereotactic needle biopsies with excellent rates of diagnostic success and acceptable morbidity relative to the needle biopsy technique. As molecular data begins to increasingly drive treatment decisions, additional biopsy techniques that afford large tissue volumes may be necessary to adapt to the new needs of pathologists and treating oncologists.
Similar content being viewed by others
Introduction
With the publication of the updated World Health Organization (WHO) grading of central nervous system tumors, molecular information has become an essential component of obtaining an integrated diagnosis. These integrated diagnoses are not merely academic—they can affect treatment decisions. Critical to modern tumor grading is the ability to obtain sufficient tissue for detailed immunohistochemistry, genetic sequencing, as well as cell culture. In the era of precision medicine, tissue availability for advanced analysis and biobanking is of paramount importance to help guide patient therapies. While open surgery to remove brain tumors generally provides sufficient tissue, deep unresectable tumors in highly eloquent locations are often sampled using navigated stereotactic needle biopsies [1,2,3,4,5,6,7]. However, these needle biopsies provide only small volume specimens and sample tissue from a single limited location. Diagnostic success with this technique can be as low as 72.1% [8, 9]. This may lead to misdiagnosis or undergrading of tumors, which has been reported to occur in 9.2–28.0% of neoplastic lesions, respectively [10, 11]. Finally, hematomas related to the procedure cannot be diagnosed and treated until after the operation [7, 12,13,14,15,16,17,18].
Recently, use of tubular retractors has been documented for the resection of deep intracranial tumors and for evacuation of subcortical hematomas and neoplastic lesions of the brainstem, basal ganglia, and ventricles [12,13,14,15,16,17, 19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. Moreover, they have increasingly become favored operative adjuncts for deep lesions given their small footprint and minimal collateral tissue damage compared to fixed blade retractors [35]. As such, this tubular retractor-based minimally invasive technique may offer the potential of improving tissue yield and diagnosis compared to needle biopsies. To compare these techniques, this retrospective analysis looked at the diagnostic accuracy and complication rates of biopsies in patients who underwent open transtubular brain biopsy at our institution between 2010 and 2018. As a comparison group, we also examined a contemporaneous group of patients undergoing stereotactic needle biopsy.
Methods
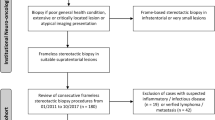
This study was approved by the Institutional Review Board at Weill Cornell Medical College. Between the years of 2010 and 2018, 18 patients underwent an open transtubular biopsy. As a control group for comparison, during the same period of time, 146 underwent stereotactic biopsy. Retrospective chart review was completed to identify demographic data, pathology specimen characteristics, pathology diagnosis versus non-diagnostic sample, postoperative surgical complications, and postoperative morbidity/mortality. Three patients who had incomplete medical records, did not have pathology reports, or whose pathology reports were from other institutions were excluded from analysis.
Patient selection and surgical technique
As this was not a prospective, randomized study, patients were selected for transtubular biopsy based on surgeon’s preference. Transtubular biopsy was offered for deep lesions that were considered “unresectable”, in that the risks of near total resection outweighed the potential oncologic benefits. For example, deep lesions in the subcortical region, insula, corpus callosum, thalamus, and/or bilateral masses (“butterfly lesions”), for which more extensive resections would likely result in post-operative neurologic deficits, were considered good candidates for transtubular biopsy. Patients presenting with recurrence of a previously treated glioma, for which tissue sampling yielded a potentially updated diagnosis and new genetic information, were also included as good candidates for a transtubular approach. These patients are generally offered a needle biopsy but given the potential for inadequate sampling and insufficient material for genetic testing, tubular biopsy is considered an alternative to provide more tissue to the pathologist. Masses that came close to the cortical surface and/or could be easily reached by a small craniotomy and cortisectomy without the need for deep dissection or neuro-navigation were not included.
The surgical technique for trans-sulcal transtubular surgery has been extensively discussed elsewhere [22, 26, 36]. The cannula should be inserted using navigational guidance along the long axis of the tumor so that the angle of insertion avoids transecting any major white matter fascicles. Diffusion tensor imaging data is generally used to plan this trajectory and fMRI is used to plan the cortical entry site, if required. In addition, as most of our tumors were gliomas, advanced imaging including perfusion analysis was used to help direct the location of the biopsy particularly in cases of minimal contrast enhancement [37,38,39]. Specifically, areas with increased blood flow or permeability were targeted, when possible, in the absence of contrast enhancement. Various tubular retractor sizes/lengths were used in this study and determined by the operating surgeon. Figure 1 demonstrates examples of tubular retractor introducers (a), trans-sulcal approach (b), and trajectory for a transtubular biopsy (c). The supplementary material video also demonstrates the introduction of the tubular retractor, visualization and biopsy of tumor, and ability to obtain hemostasis prior to removing the retractor.
For statistical analysis, continuous variables were compared using homoscedastic t tests and Wilcoxon rank sum tests. Since the cohort of patients who underwent transtubular biopsy was relatively small, categorical variables were compared as contingency tables using Fisher’s exact tests. A p-value of < 0.05 was considered statistically significant. Statistical analysis was performed using Microsoft Excel, MATLAB, and Stata statistical software.
Results
Demographic data regarding patients in stereotactic biopsy and transtubular biopsy cohorts is presented in Table 1. Of note, there was no statistical difference between mean age or gender. Temporal lobe lesions were more frequently sampled by transtubular compared to stereotactic biopsy (p = 0.04). Tumors and specifically gliomas were statistically the more common diagnosis in the transtubular group, likely due to the inclusion bias of choosing the transtubular approach for cases where genetic testing was required for further treatment decisions.
Tubular biopsies resulted in a higher volume of tissue provided to the pathologist than needle biopsies (1.26 cm3 vs. 0.3 cm3; p < 0.0001). Moreover, while all transtubular biopsies obtained diagnostic frozen sections for testing, only 82.1% of stereotactic biopsies obtained a frozen section (p = 0.046). While all tubular biopsies resulted in a permanent section for testing, only 95.2% of needle biopsies provided a permanent specimen (ns). Finally, there was a higher rate of non-diagnostic biopsy with stereotactic biopsy (n = 19) specimens compared to transtubular biopsies (n = 0; p = 0.13). Six patients who underwent stereotactic biopsy required reoperation for diagnosis, while none of the patients who underwent transtubular biopsy required reoperation in order to obtain a diagnostic permanent specimen (Table 2).
Three (2.1%) patients who underwent stereotactic biopsy experienced post-biopsy hemorrhage that extended outside of the stereotactic needle tract compared to one (5.6%) in the transtubular group (p = 0.37). None of the patients found to have a postoperative hematoma required subsequent operative evacuation.
Case illustration
A 47-year-old female with a history of neurofibromatosis type 1 (NF1) and previously resected pilocytic astrocytoma during childhood had been followed by serial MRI for a suspected low-grade glioma of the right thalamus. On repeat MRI, the patient’s lesion was found to have increased in size and volume of enhancement (Fig. 1). A transtubular biopsy of the lesion was planned and the patient was placed in the lateral position and a small craniotomy was made for insertion of the BrainPath (Nico, IN) into the right ventricle. Through the tubular retractor, a sample of the tumor was obtained and the tumor was partially debulked to mitigate against future compartmentalized hydrocephalus. Histopathology demonstrated IDH negative WHO grade III astrocytoma, and a sample of tumor was sent for comprehensive genomic sequencing (FoundationOne, Foundation Medicine, MA). Post-operatively, the patient recovered without complication and completed concurrent radiation and temozolomide treatment. After two cycles of adjuvant temozolomide, imaging showed central necrosis of the thalamic mass and a new enhancing lesion in the left uncus, which were confirmed to likely represent active tumor with fluorodeoxyglucose positron emission tomography (FDG-PET). At that time, the decision was made to use a PD-1 inhibitor, nivolumab (Opdivo, Bristol-Myers Squibb), instead of CCNU/bevacizumab because the tumor genetic analysis was notable for broad amplifications in the regions encoding CD274 (which encodes PD-L1) and PDCD1LG2 (which encodes PD-L2), thus rendering the tumor potentially susceptible to checkpoint inhibition.
Subsequent MRI 2 months after beginning nivolumab therapy demonstrated initial increase in size of necrotic thalamic mass (felt to be treatment effect) but decrease in size of contralateral areas of nodular enhancement (Fig. 2). Nivolumab treatment was continued and over the following 6 months repeat MRIs demonstrated stability of the right thalamic lesion. Ultimately, 6 months after initiating nivolumab, there was progression of the tumor with new enhancing nodules along the left thalamus. This case demonstrates the utility of advance tissue diagnostics facilitated by large tissue volumes which can directly affect treatment decisions (Fig. 3).
Discussion
Here we describe the first comparison of outcomes between stereotactic and transtubular brain biopsy. Notably, the complication profiles of both techniques were similar. Early reports of stereotactic biopsy demonstrated that the technique yielded relatively few surgical complications. However, the main drawbacks of stereotactic biopsy include significant rates of misdiagnosis and undergrading, particularly of glial tumors. Our goal was to assess whether transtubular biopsies offer a similar safety profile while also improving the rate of diagnosis.
The 13% rate of non-diagnostic biopsy and 4% rate of needing repeat surgery in our series suggested room for improvement in stereotactic biopsy sampling. While our transtubular group remains small and thus limits our ability to demonstrate a statistical difference, our data indicate a 100% successful diagnosis rate with transtubular biopsies with significantly more available tissue available (~ 4x) for postoperative molecular analysis. Needle specimens often produce multiple cores with necrotic tissue, potentially limiting the range of diagnostic tests that can be performed on the tissue (especially with high-grade gliomas). With the publication of the updated World Health Organization (WHO) grading of central nervous system tumors, molecular information has become an essential component of obtaining an integrated diagnosis. These integrated diagnoses are not merely academic—they can affect treatment decisions as evidenced by the above case illustration. As tumor neuropathology continues to advance, the testing required to further refine diagnoses will only increase, thereby requiring even more tissue for analysis. Our data indicate that transtubular biopsies are effective in this regard. Specifically, in addition to accurate diagnosis, transtubular biopsies generate a significantly increased volume of tumor. While multiple needle cores and trajectories can increase tissue yield for the stereotactic needle approach, each pass with the biopsy needle incurs further risk of iatrogenic injury, which cannot be diagnosed until post-operative imaging is obtained or the patient is examined after surgery. Furthermore, with increasing evidence for intratumoral heterogeneity, the need to sample multiple regions of a tumor may become more common [40, 41]. With the transtubular approach, a single trajectory and pass of the retractor through the long axis of a tumor can allow for multiple samples from different depths along that chosen trajectory. Moreover hemostasis can be achieved under direct visualization. Finally, re-operations were not required among the transtubular group to obtain a diagnosis; this has a likely positive effect on medical costs, patient satisfaction and comfort.
The safety of this approach from this pilot data is reassuring given that there was not a significant difference in the rates of hemorrhage in the transtubular and stereotactic biopsy cohorts. Although it is more invasive, transtubular biopsy allows surgeons to obtain hemostasis under direct vision. Moreover, the rates of neurologic morbidity were acceptable in the transtubular group compared to the stereotactic biopsy group. The one hemorrhage in the transtubular group was not clinically meaningful and was observed. Previous studies have identified several risk factors for postoperative complications from stereotactic biopsy, including preoperative pharmacologic therapy, lesion locus and histology, and surgeon experience [7, 18, 42, 43]. While each biopsy system possesses unique drawbacks, there appear to be no significant differences in the rates of complications between stereotactic biopsy and minimally invasive open transtubular biopsy. However, these conclusions are tempered and limited by the small sample size of the transtubular group, and requires continued re-assessment as the procedure becomes more commonly used. We suggest that transtubular biopsies be considered with any subcortical lesion. In particular, diffuse tumors with little to no contrast enhancement may uniquely benefit from the extensive tissue generated from this technique. These latter cases are most prone to misdiagnosis or undergrading and therefore mostly likely to benefit from larger tissue volumes.
This study is limited in our ability to make conclusions based on the small sample size of the tubular retractor cohort. This reflects our belief that the utility of this technique is confined to subcortical lesions where extensive tissue may be of clinical value. A prospective, randomized comparison of the techniques would also be required to best compare the outcomes in these patients. There is likely a selection bias in patients that were sampled using tubular retractors in this study. Although the above findings related to rate of non-diagnostic biopsies did not yield statistical significance due to the sample size, these preliminary results provide compelling grounds for future examination of diagnostic efficacy and patient outcomes following tubular retractor biopsies. Finally, there remains uncertainty on the value of precision medicine and precision oncology writ large [44]. The number of mutations that have approved drug targets are limited. Indeed, the pace at which our ability to accrue high-resolution molecular information about individual tumors has far outpaced our ability to act on this information in a clinically meaningful way [45]. For the patient with a targetable mutation, though, such personalized information can be disease altering. At the programmatic level, however, it remains to be seen how the resources required for precision medicine programs can be balanced against the knowledge that few patients will directly benefit from sequencing data at present. Studies like SHIVA have not shown population level benefits from precision oncology approaches versus standard of care [46]. Such foundational data and “growing pains” though are essential if oncology is to take advantage of the mutations identified through precision medicine pipelines. Collaboration with industry and federal funding sources will be essential to design “n of 1” clinical trial designs that take advantage of precision oncology data.
Conclusions
Transtubular biopsies are a viable alternative to stereotactic needle biopsies. The rates of diagnostic success are excellent with acceptable morbidity relative to the needle biopsy technique. Future studies should include larger cohorts and help advance the discussion regarding optimal patient selection. Finally, technologic improvements might include a smaller diameter tube and better instrumentation for utilization through the narrow transtubular corridor.
References
Rahman M, Murad GJA, Mocco J (2009) Early history of the stereotactic apparatus in neurosurgery. Neurosurg Focus 27:E12. https://doi.org/10.3171/2009.7.FOCUS09118
Krieger MD, Chandrasoma PT, Zee C-S, Apuzzo MLJ (1998) Role of stereotactic biopsy in the diagnosis and management of brain tumors. Semin Surg Oncol 14:13–25. https://doi.org/10.1002/(SICI)1098-2388(199801/02)14:1%3C13::AID-SSU3%3E3.0.CO;2-5
Kickingereder P, Willeit P, Simon T, Ruge MI (2013) Diagnostic value and safety of stereotactic biopsy for brainstem tumors: a systematic review and meta-analysis of 1480 cases. Neurosurgery 72:873–881. https://doi.org/10.1227/NEU.0b013e31828bf445 (discussion 882; quiz 882)
Reithmeier T, Lopez WO, Doostkam S et al (2013) Intraindividual comparison of histopathological diagnosis obtained by stereotactic serial biopsy to open surgical resection specimen in patients with intracranial tumours. Clin Neurol Neurosurg 115:1955–1960. https://doi.org/10.1016/j.clineuro.2013.05.019
Lu Y, Yeung C, Radmanesh A et al (2015) Comparative effectiveness of frame-based, frameless, and intraoperative magnetic resonance imaging-guided brain biopsy techniques. World Neurosurg 83:261–268. https://doi.org/10.1016/j.wneu.2014.07.043
Hall WA (1998) The safety and efficacy of stereotactic biopsy for intracranial lesions. Cancer 82:1749–1755. https://doi.org/10.1002/(SICI)1097-0142(19980501)82:9%3C1756::AID-CNCR23%3E3.0.CO;2-2
Dammers R, Haitsma IK, Schouten JW et al (2008) Safety and efficacy of frameless and frame-based intracranial biopsy techniques. Acta Neurochir (Wien) 150:23–29. https://doi.org/10.1007/s00701-007-1473-x
Khatab S, Spliet W, Woerdeman PA (2014) Frameless image-guided stereotactic brain biopsies: emphasis on diagnostic yield. Acta Neurochir (Wien) 156:1441–1450. https://doi.org/10.1007/s00701-014-2145-2
Castle M, Nájera E, Samprón N et al (2014) Frameless stereotactic biopsy: diagnostic yield and complications. Neurocirugia (Asturias) 25:56–61. https://doi.org/10.1016/j.neucir.2013.11.003
Muragaki Y, Chernov M, Maruyama T et al (2008) Low-grade glioma on stereotactic biopsy: how often is the diagnosis accurate? Minim Invasive Neurosurg MIN 51:275–279. https://doi.org/10.1055/s-0028-1082322
Slowiński J, Harabin-Slowińska M, Mrówka R (1999) Smear technique in the intra-operative brain tumor diagnosis: its advantages and limitations. Neurol Res 21:121–124
Herrera SR, Shin JH, Chan M et al (2010) Use of transparent plastic tubular retractor in surgery for deep brain lesions: a case series. Surg Technol Int 19:47–50
Recinos PF, Raza SM, Jallo GI, Recinos VR (2011) Use of a minimally invasive tubular retraction system for deep-seated tumors in pediatric patients. J Neurosurg Pediatr 7:516–521. https://doi.org/10.3171/2011.2.PEDS10515
Jo K-W, Shin HJ, Nam D-H et al (2011) Efficacy of endoport-guided endoscopic resection for deep-seated brain lesions. Neurosurg Rev 34:457–463. https://doi.org/10.1007/s10143-011-0319-4
Ichinose T, Goto T, Morisako H et al (2010) Microroll retractor for surgical resection of brainstem cavernomas. World Neurosurg 73:520–522. https://doi.org/10.1016/j.wneu.2010.06.049
Ratre S, Yadav YR, Parihar VS, Kher Y (2016) Microendoscopic removal of deep-seated brain tumors using tubular retraction system. J Neurol Surg A 77:312–320. https://doi.org/10.1055/s-0036-1580595
Jo K-I, Chung SB, Jo K-W et al (2011) Microsurgical resection of deep-seated lesions using transparent tubular retractor: pediatric case series. Childs Nerv Syst 27:1989–1994. https://doi.org/10.1007/s00381-011-1529-3
Kreth FW, Muacevic A, Medele R et al (2001) The risk of haemorrhage after image guided stereotactic biopsy of intra-axial brain tumours–a prospective study. Acta Neurochir (Wien) 143:539–545 (discussion 545–546)
Akai T, Shiraga S, Sasagawa Y et al (2008) Intra-parenchymal tumor biopsy using neuroendoscopy with navigation. Minim Invasive Neurosurg MIN 51:83–86. https://doi.org/10.1055/s-2007-1004562
Almenawer SA, Crevier L, Murty N et al (2013) Minimal access to deep intracranial lesions using a serial dilatation technique: case-series and review of brain tubular retractor systems. Neurosurg Rev 36:321–330. https://doi.org/10.1007/s10143-012-0442-x
Fahim DK, Relyea K, Nayar VV et al (2009) Transtubular microendoscopic approach for resection of a choroidal arteriovenous malformation. J Neurosurg Pediatr 3:101–104. https://doi.org/10.3171/2008.11.PEDS08280
Greenfield JP, Cobb WS, Tsouris AJ, Schwartz TH (2008) Stereotactic minimally invasive tubular retractor system for deep brain lesions. Neurosurgery 63:334–339. https://doi.org/10.1227/01.neu.0000334741.61745.72 (discussion 339–340)
Jho H-D, Alfieri A (2002) Endoscopic removal of third ventricular tumors: a technical note. Minim Invasive Neurosurg MIN 45:114–119. https://doi.org/10.1055/s-2002-32487
Kassam AB, Engh JA, Mintz AH, Prevedello DM (2009) Completely endoscopic resection of intraparenchymal brain tumors. J Neurosurg 110:116–123. https://doi.org/10.3171/2008.7.JNS08226
Kelly PJ (1989) Future perspectives in stereotactic neurosurgery: stereotactic microsurgical removal of deep brain tumors. J Neurosurg Sci 33:149–154
Kelly PJ, Goerss SJ, Kall BA (1988) The stereotaxic retractor in computer-assisted stereotaxic microsurgery. Technical note. J Neurosurg 69:301–306. https://doi.org/10.3171/jns.1988.69.2.0301
Moshel YA, Link MJ, Kelly PJ (2007) Stereotactic volumetric resection of thalamic pilocytic astrocytomas. Neurosurgery 61:66–75. https://doi.org/10.1227/01.neu.0000279725.13521.a3 (discussion 75)
Nishihara T, Nagata K, Tanaka S et al (2005) Newly developed endoscopic instruments for the removal of intracerebral hematoma. Neurocrit Care 2:67–74. https://doi.org/10.1385/NCC:2:1:067
Otsuki T, Jokura H, Yoshimoto T (1990) Stereotactic guiding tube for open-system endoscopy: a new approach for the stereotactic endoscopic resection of intra-axial brain tumors. Neurosurgery 27:326–330
Patil A-A (1987) Stereotactic excision of deep brain lesions using probe guided brain retractor. Acta Neurochir (Wien) 87:150–152. https://doi.org/10.1007/BF01476067
Raza SM, Recinos PF, Avendano J et al (2011) Minimally invasive trans-portal resection of deep intracranial lesions. Minim Invasive Neurosurg MIN 54:5–11. https://doi.org/10.1055/s-0031-1273734
Ross DA (1993) A simple stereotactic retractor for use with the Leksell stereotactic system. Neurosurgery 32:475–476 (discussion 476)
Constantini S, Mohanty A, Zymberg S et al (2013) Safety and diagnostic accuracy of neuroendoscopic biopsies: an international multicenter study. J Neurosurg Pediatr 11:704–709. https://doi.org/10.3171/2013.3.PEDS12416
Chrastina J, Novak Z, Riha I et al (2014) Diagnostic value of brain tumor neuroendoscopic biopsy and correlation with open tumor resection. J Neurol Surg A 75:110–115. https://doi.org/10.1055/s-0032-1320032
Bander ED, Jones SH, Kovanlikaya I, Schwartz TH (2016) Utility of tubular retractors to minimize surgical brain injury in the removal of deep intraparenchymal lesions: a quantitative analysis of FLAIR hyperintensity and apparent diffusion coefficient maps. J Neurosurg 124:1053–1060. https://doi.org/10.3171/2015.4.JNS142576
Day JD (2017) Transsulcal parafascicular surgery using brain path® for subcortical lesions. Neurosurgery 64:151–156. https://doi.org/10.1093/neuros/nyx324
Moussazadeh N, Tsiouris AJ, Ramakrishna R (2016) Advanced imaging for biopsy guidance in primary brain tumors. Cureus. https://doi.org/10.7759/cureus.504
Chiang GC, Kovanlikaya I, Choi C et al (2018) Magnetic resonance spectroscopy, positron emission tomography and radiogenomics—relevance to glioma. Front Neurol. https://doi.org/10.3389/fneur.2018.00033
Salama GR, Heier LA, Patel P et al (2018) Diffusion weighted/tensor imaging, functional MRI and perfusion weighted imaging in glioblastoma—foundations and future. Front Neurol. https://doi.org/10.3389/fneur.2017.00660
Sottoriva A, Spiteri I, Piccirillo SGM et al (2013) Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci 110:4009–4014. https://doi.org/10.1073/pnas.1219747110
Patel AP, Tirosh I, Trombetta JJ et al (2014) Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344:1396–1401. https://doi.org/10.1126/science.1254257
Warnick RE, Longmore LM, Paul CA, Bode LA (2003) Postoperative management of patients after stereotactic biopsy: results of a survey of the AANS/CNS section on tumors and a single institution study. J Neurooncol 62:289–296
Favre J, Taha JM, Burchiel KJ (2002) An analysis of the respective risks of hematoma formation in 361 consecutive morphological and functional stereotactic procedures. Neurosurgery 50:48–56 (discussion 56–57)
Tannock IF, Hickman JA (2016) Limits to personalized cancer medicine. N Engl J Med 375:1289–1294. https://doi.org/10.1056/NEJMsb1607705
Pisapia DJ, Magge R, Ramakrishna R (2017) Improved pathologic diagnosis—forecasting the future in glioblastoma. Front Neurol. https://doi.org/10.3389/fneur.2017.00707
Tourneau CL, Delord J-P, Gonçalves A et al (2015) Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol 16:1324–1334. https://doi.org/10.1016/S1470-2045(15)00188-6
Acknowledgements
The authors would like to acknowledge Debra D’Angelo for her contributions to the statistical analyses performed in this research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material video (MP4 19628 KB)
Rights and permissions
About this article
Cite this article
Bander, E.D., Jones, S.H., Pisapia, D. et al. Tubular brain tumor biopsy improves diagnostic yield for subcortical lesions. J Neurooncol 141, 121–129 (2019). https://doi.org/10.1007/s11060-018-03014-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-03014-w