Abstract
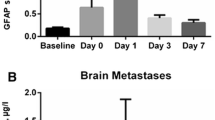
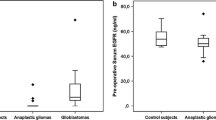
Recent studies identified serum concentrations of the astroglial protein glial fibrillary acidic protein (GFAP) to be indicative of glioblastoma (GBM) in patients with newly diagnosed space occupying cerebral mass lesions. Until now, no data is available whether GFAP serum concentrations decrease after first therapy and whether GFAP may be used as a predictor of survival and an indicator of tumor recurrence. In this prospective study, we included 44 patients with a single space occupying cerebral mass lesion suspicious for GBM. GBM was histopathologically proven in 33 cases. After initial therapy, patients were followed up until tumor recurrence (defined according to the RANO criteria) or death (maximum observation period 78 weeks). Blood was sampled on a regular basis, and GFAP serum levels were determined using an immunofluorescence assay. Prior to any intervention, 14 of the 33 GBM patients had elevated GFAP serum concentrations (median 0.25 µg/L, interquartile range 0.13–0.53), whereas only one out of 11 patients having other tumor entities revealed a slightly increased GFAP serum level (0.06 µg/L). Following surgery (i.e., biopsy, full or partial resection), all initially GFAP positive GBM patients showed decreased serum concentrations. During the follow-up period, we found a minimal GFAP increase in one patient only (0.04 µg/L; week 52), although 23 out of 31 available GBM patients developed tumor progression or died. No difference was found regarding the survival rate and the time to tumor recurrence between initially GFAP positive and GFAP negative GBM patients. In GBM patients, initially elevated GFAP serum concentrations decrease after the first diagnostic or therapeutic intervention. GFAP was not predictive for tumor recurrence.


Similar content being viewed by others
Abbreviations
- Approx.:
-
Approximately
- CCNU:
-
Lomustine
- CSF:
-
Cerebrospinal fluid
- CNS:
-
Central nervous system
- GBM:
-
Glioblastoma
- GFAP:
-
Glial fibrillary acidic protein
- Gy:
-
Gray
- FLAIR:
-
Fluid attenuated inversion recovery
- IDH1:
-
Isocitrate dehydrogenase 1
- MGMT:
-
O-6-Methylguanine-DNA methyltransferase
- MRI:
-
Magnet resonance imaging
- OP:
-
Operation
- PD:
-
Progressive disease
- PNET:
-
Primitive neuroectodermal tumor
- RANO:
-
Response assessment in neuro-oncology
- TMZ:
-
Temozolomide
- WHO:
-
World Health Organization
References
Bignami A, Eng LF, Dahl D, Uyeda CT (1972) Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res 43:429–435
Eng LF, Ghirnikar RS, Lee YL (2000) Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochem Res 25:1439–1451
Brunkhorst R, Pfeilschifter W, Foerch C (2010) Astroglial proteins as diagnostic markers of acute intracerebral hemorrhage-pathophysiological background and clinical findings. Transl Stroke Res 1:246–251. doi:10.1007/s12975-010-0040-6
Mondello S, Papa L, Buki A, Bullock MR, Czeiter E, Tortella FC, Wang KK, Hayes RL (2011) Neuronal and glial markers are differently associated with computed tomography findings and outcome in patients with severe traumatic brain injury: a case control study. Crit Care 15:R156. doi:10.1186/cc10286
Lumpkins KM, Bochicchio GV, Keledjian K, Simard JM, McCunn M, Scalea T (2008) Glial fibrillary acidic protein is highly correlated with brain injury. J Trauma 65: 778–782. doi:10.1097/TA.0b013e318185db2d (discussion 782–774)
Luger S, Witsch J, Dietz A, Hamann GF, Minnerup J, Schneider H, Sitzer M, Wartenberg KE, Niessner M, Foerch C, BE FAST II and the IGNITE Study Groups (2017) Glial fibrillary acidic protein serum levels distinguish between intracerebral hemorrhage and cerebral ischemia in the early phase of stroke. Clin Chem 63:377–385. doi:10.1373/clinchem.2016.263335
Foerch C, Niessner M, Back T, Bauerle M, De Marchis GM, Ferbert A, Grehl H, Hamann GF, Jacobs A, Kastrup A, Klimpe S, Palm F, Thomalla G, Worthmann H, Sitzer M, Group BFS (2012) Diagnostic accuracy of plasma glial fibrillary acidic protein for differentiating intracerebral hemorrhage and cerebral ischemia in patients with symptoms of acute stroke. Clin Chem 58:237–245. doi:10.1373/clinchem.2011.172676
Tichy J, Spechtmeyer S, Mittelbronn M, Hattingen E, Rieger J, Senft C, Foerch C (2016) Prospective evaluation of serum glial fibrillary acidic protein (GFAP) as a diagnostic marker for glioblastoma. J Neurooncol 126:361–369. doi:10.1007/s11060-015-1978-8
Jung CS, Foerch C, Schanzer A, Heck A, Plate KH, Seifert V, Steinmetz H, Raabe A, Sitzer M (2007) Serum GFAP is a diagnostic marker for glioblastoma multiforme. Brain 130:3336–3341. doi:10.1093/brain/awm263
Gallego Perez-Larraya J, Paris S, Idbaih A, Dehais C, Laigle-Donadey F, Navarro S, Capelle L, Mokhtari K, Marie Y, Sanson M, Hoang-Xuan K, Delattre JY, Mallet A (2014) Diagnostic and prognostic value of preoperative combined GFAP, IGFBP-2, and YKL-40 plasma levels in patients with glioblastoma. Cancer 120:3972–3980. doi:10.1002/cncr.28949
Brommeland T, Rosengren L, Fridlund S, Hennig R, Isaksen V (2007) Serum levels of glial fibrillary acidic protein correlate to tumour volume of high-grade gliomas. Acta Neurol Scand 116:380–384. doi:10.1111/j.1600-0404.2007.00889.x
Finn MA, Blumenthal DT, Salzman KL, Jensen RL (2007) Transient postictal MRI changes in patients with brain tumors may mimic disease progression. Surg Neurol 67: 246–250. doi:10.1016/j.surneu.2006.04.015 (discussion 250)
Kumar AJ, Leeds NE, Fuller GN, Van Tassel P, Maor MH, Sawaya RE, Levin VA (2000) Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 217:377–384. doi:10.1148/radiology.217.2.r00nv36377
Hygino da Cruz LC Jr, Rodriguez I, Domingues RC, Gasparetto EL, Sorensen AG (2011) Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am J Neuroradiol 32:1978–1985. doi:10.3174/ajnr.A2397
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972. doi:10.1200/JCO.2009.26.3541
Kiviniemi A, Gardberg M, Frantzen J, Parkkola R, Vuorinen V, Pesola M, Minn H (2015) Serum levels of GFAP and EGFR in primary and recurrent high-grade gliomas: correlation to tumor volume, molecular markers, and progression-free survival. J Neurooncol 124:237–245. doi:10.1007/s11060-015-1829-7
Husain H, Savage W, Grossman SA, Ye X, Burger PC, Everett A, Bettegowda C, Diaz LA Jr, Blair C, Romans KE, Holdhoff M (2012) Pre- and post-operative plasma glial fibrillary acidic protein levels in patients with newly diagnosed gliomas. J Neurooncol 109:123–127. doi:10.1007/s11060-012-0874-8
Gurses C, Ekizoglu O, Orhan N, Ustek D, Arican N, Ahishali B, Elmas I, Kucuk M, Bilgic B, Kemikler G, Kalayci R, Karadeniz A, Kaya M (2009) Levetiracetam decreases the seizure activity and blood-brain barrier permeability in pentylenetetrazole-kindled rats with cortical dysplasia. Brain Res 1281:71–83. doi:10.1016/j.brainres.2009.05.033
Ostergaard L, Hochberg FH, Rabinov JD, Sorensen AG, Lev M, Kim L, Weisskoff RM, Gonzalez RG, Gyldensted C, Rosen BR (1999) Early changes measured by magnetic resonance imaging in cerebral blood flow, blood volume, and blood-brain barrier permeability following dexamethasone treatment in patients with brain tumors. J Neurosurg 90:300–305. doi:10.3171/jns.1999.90.2.0300
Kim JH, Bae Kim Y, Han JH, Cho KG, Kim SH, Sheen SS, Lee HW, Jeong SY, Kim BY, Lee KB (2012) Pathologic diagnosis of recurrent glioblastoma: morphologic, immunohistochemical, and molecular analysis of 20 paired cases. Am J Surg Pathol 36:620–628. doi:10.1097/PAS.0b013e318246040c
Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95:190–198. doi:10.3171/jns.2001.95.2.0190
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: JV, JT, CF. Performed the experiments: JV, MW. Analyzed the data: JV, JT, JR, CF. Contributed reagents, materials, financial support: CF. Wrote the paper: JV, JT, JR, CF. Supervisor of the study: CF. Corrected and approved the final version of the manuscript: all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Vietheer, JM., Rieger, J., Wagner, M. et al. Serum concentrations of glial fibrillary acidic protein (GFAP) do not indicate tumor recurrence in patients with glioblastoma. J Neurooncol 135, 193–199 (2017). https://doi.org/10.1007/s11060-017-2565-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2565-y