Abstract
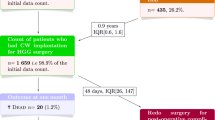
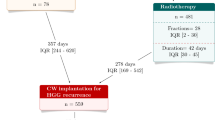
For newly diagnosed glioblastomas treated with resection in association with the standard combined chemoradiotherapy, the impact of Carmustine wafer implantation remains debated regarding postoperative infections, quality of life, and feasibility of adjuvant oncological treatments. To assess together safety, tolerance and efficacy of Carmustine wafer implantation and of extent of resection for glioblastoma patients in real-life experience. Observational retrospective monocentric study including 340 consecutive adult patients with a newly diagnosed supratentorial glioblastoma who underwent surgical resection with (n = 123) or without (n = 217) Carmustine wafer implantation as first-line oncological treatment. Carmustine wafer implantation and extent of resection did not significantly increase postoperative complications, including postoperative infections (p = 0.269, and p = 0.446, respectively). Carmustine wafer implantation and extent of resection did not significantly increase adverse events during adjuvant oncological therapies (p = 0.968, and p = 0.571, respectively). Carmustine wafer implantation did not significantly alter the early postoperative Karnofsky performance status (p = 0.402) or the Karnofsky performance status after oncological treatment (p = 0.636) but a subtotal or total surgical resection significantly improved those scores (p < 0.001, and p < 0.001, respectively). Carmustine wafer implantation, subtotal and total resection, and standard combined chemoradiotherapy were independently associated with longer event-free survival (adjusted Hazard Ratio (aHR), 0.74 [95% CI 0.55–0.99], p = 0.043; aHR, 0.70 [95% CI 0.54–0.91], p = 0.009; aHR, 0.40 [95% CI 0.29–0.55], p < 0.001, respectively) and with longer overall survival (aHR, 0.69 [95% CI 0.49–0.96], p = 0.029; aHR, 0.52 [95% CI 0.38–0.70], p < 0.001; aHR, 0.58 [95% CI 0.42–0.81], p = 0.002, respectively). Carmustine wafer implantation in combination with maximal resection, followed by standard combined chemoradiotherapy is safe, efficient, and well-tolerated in newly diagnosed supratentorial glioblastomas in adults.

Similar content being viewed by others
Abbreviations
- CI:
-
Confidence interval
- HR:
-
Hazard ratio
- KPS:
-
Karnofsky performance status
- MRI:
-
Magnetic resonance imaging
- OS:
-
Overall survival
- EFS:
-
Event-free survival
- RPA:
-
Recursive partitioning analysis
- RTOG:
-
Radiation Therapy Oncology Group
- WHO:
-
World Health Organization
References
Ricard D, Idbaih A, Ducray F, et al (2012) Primary brain tumours in adults. Lancet Lond Engl 379:1984–1996. doi:10.1016/S0140-6736(11)61346-9
Marko NF, Weil RJ, Schroeder JL et al (2014) Extent of resection of glioblastoma revisited: personalized survival modeling facilitates more accurate survival prediction and supports a maximum-safe-resection approach to surgery. J Clin Oncol Off J Am Soc Clin Oncol 32:774–782. doi:10.1200/JCO.2013.51.8886
Sanai N, Polley M-Y, McDermott MW et al (2011) An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115:3–8. doi:10.3171/2011.2.JNS10998
Lacroix M, Abi-Said D, Fourney DR et al (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95:190–198. doi:10.3171/jns.2001.95.2.0190
Li YM, Suki D, Hess K, Sawaya R (2016) The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection? J Neurosurg 124:977–988. doi:10.3171/2015.5.JNS142087
Pallud J, Audureau E, Noel G, et al (2015) Long-term results of carmustine wafer implantation for newly diagnosed glioblastomas: a controlled propensity-matched analysis of a French multicenter cohort. Neuro-Oncol 17:1609–1619. doi:10.1093/neuonc/nov126
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466. doi:10.1016/S1470-2045(09)70025-7
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. doi:10.1056/NEJMoa043330
Brem H, Piantadosi S, Burger PC, et al (1995) Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet Lond Engl 345:1008–1012.
Westphal M, Hilt DC, Bortey E, et al (2003) A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-Oncol 5:79–88. doi:10.1215/S1522-8517-02-00023-6
McGirt MJ, Than KD, Weingart JD et al (2009) Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg 110:583–588. doi:10.3171/2008.5.17557
Attenello FJ, Mukherjee D, Datoo G et al (2008) Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: a 10-year institutional experience. Ann Surg Oncol 15:2887–2893. doi:10.1245/s10434-008-0048-2
Valtonen S, Timonen U, Toivanen P et al (1997) Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: a randomized double-blind study. Neurosurgery 41:44-48-49
Westphal M, Ram Z, Riddle V, et al (2006) Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir (Wien) 148:269–275; discussion 275. doi:10.1007/s00701-005-0707-z
Duntze J, Litré C-F, Eap C et al (2013) Implanted carmustine wafers followed by concomitant radiochemotherapy to treat newly diagnosed malignant gliomas: prospective, observational, multicenter study on 92 cases. Ann Surg Oncol 20:2065–2072. doi:10.1245/s10434-012-2764-x
Affronti ML, Heery CR, Herndon JE et al (2009) Overall survival of newly diagnosed glioblastoma patients receiving carmustine wafers followed by radiation and concurrent temozolomide plus rotational multiagent chemotherapy. Cancer 115:3501–3511. doi:10.1002/cncr.24398
Menei P, Metellus P, Parot-Schinkel E et al (2010) Biodegradable carmustine wafers (Gliadel) alone or in combination with chemoradiotherapy: the French experience. Ann Surg Oncol 17:1740–1746. doi:10.1245/s10434-010-1081-5
La Rocca RV, Mehdorn HM (2009) Localized BCNU chemotherapy and the multimodal management of malignant glioma. Curr Med Res Opin 25:149–160. doi:10.1185/03007990802611935
Pan E, Mitchell SB, Tsai JS (2008) A retrospective study of the safety of BCNU wafers with concurrent temozolomide and radiotherapy and adjuvant temozolomide for newly diagnosed glioblastoma patients. J Neurooncol 88:353–357. doi:10.1007/s11060-008-9576-7
Pavlov V, Page P, Abi-Lahoud G et al (2015) Combining intraoperative carmustine wafers and Stupp regimen in multimodal first-line treatment of primary glioblastomas. Br J Neurosurg 29:524–531. doi:10.3109/02688697.2015.1012051
Mirimanoff R-O, Gorlia T, Mason W et al (2006) Radiotherapy and temozolomide for newly diagnosed glioblastoma: recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 phase III randomized trial. J Clin Oncol Off J Am Soc Clin Oncol 24:2563–2569. doi:10.1200/JCO.2005.04.5963
Vogelbaum MA, Jost S, Aghi MK et al (2012) Application of novel response/progression measures for surgically delivered therapies for gliomas: Response Assessment in Neuro-Oncology (RANO) Working Group. Neurosurgery 70:234-243-244. doi:10.1227/NEU.0b013e318223f5a7
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol Off J Am Soc Clin Oncol 28:1963–1972. doi:10.1200/JCO.2009.26.3541
Aoki T, Nishikawa R, Sugiyama K et al (2014) A multicenter phase I/II study of the BCNU implant (Gliadel(®) Wafer) for Japanese patients with malignant gliomas. Neurol Med Chir (Tokyo) 54:290–301
Lawson HC, Sampath P, Bohan E et al (2007) Interstitial chemotherapy for malignant gliomas: the Johns Hopkins experience. J Neurooncol 83:61–70. doi:10.1007/s11060-006-9303-1
Ashby LS, Smith KA, Stea B (2016) Gliadel wafer implantation combined with standard radiotherapy and concurrent followed by adjuvant temozolomide for treatment of newly diagnosed high-grade glioma: a systematic literature review. World J Surg Oncol 14:225. doi:10.1186/s12957-016-0975-5
Sai K, Zhong M-G, Wang J et al (2014) Safety evaluation of high-dose BCNU-loaded biodegradable implants in Chinese patients with recurrent malignant gliomas. J Neurol Sci 343:60–65. doi:10.1016/j.jns.2014.05.022
Samis Zella MA, Wallocha M, Slotty PJ et al (2014) Evaluation of post-operative complications associated with repeat resection and BCNU wafer implantation in recurrent glioblastoma. Acta Neurochir (Wien) 156:313–323. doi:10.1007/s00701-013-1931-6
Barr JG, Grundy PL (2012) The effects of the NICE Technology Appraisal 121 (gliadel and temozolomide) on survival in high-grade glioma. Br J Neurosurg 26:818–822. doi:10.3109/02688697.2012.697221
De Bonis P, Anile C, Pompucci A et al (2012) Safety and efficacy of Gliadel wafers for newly diagnosed and recurrent glioblastoma. Acta Neurochir (Wien) 154:1371–1378. doi:10.1007/s00701-012-1413-2
Subach BR, Witham TF, Kondziolka D et al (1999) Morbidity and survival after 1,3-bis(2-chloroethyl)-1-nitrosourea wafer implantation for recurrent glioblastoma: a retrospective case-matched cohort series. Neurosurgery 45:17-22-23
Salmaggi A, Milanesi I, Silvani A et al (2013) Prospective study of carmustine wafers in combination with 6-month metronomic temozolomide and radiation therapy in newly diagnosed glioblastoma: preliminary results. J Neurosurg 118:821–829. doi:10.3171/2012.12.JNS111893
Sacko A, Hou M-M, Temgoua M et al (2015) Evolution of the Karnosky Performance Status throughout life in glioblastoma patients. J Neurooncol 122:567–573. doi:10.1007/s11060-015-1749-6
McGirt MJ, Chaichana KL, Attenello FJ et al (2008) Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery 63:700-707-708. doi:10.1227/01.NEU.0000325729.41085.73
Miglierini P, Bouchekoua M, Rousseau B et al (2012) Impact of the per-operatory application of GLIADEL wafers (BCNU, carmustine) in combination with temozolomide and radiotherapy in patients with glioblastoma multiforme: efficacy and toxicity. Clin Neurol Neurosurg 114:1222–1225. doi:10.1016/j.clineuro.2012.02.056
Chowdhary SA, Ryken T, Newton HB (2015) Survival outcomes and safety of carmustine wafers in the treatment of high-grade gliomas: a meta-analysis. J Neurooncol 122:367–382. doi:10.1007/s11060-015-1724-2
Acknowledgements
These physicians are greatly acknowledged (in alphabetical order): Felipe ANDREIUOLO, Céline BOTELLA, Karl CHAMPEAUX, Francine CHASSOUX, Myriam EDJLALI-GOUJON, Raphaël GAILLARD, Sylvie GODON-HARDY, Maria KOZIAK, Elisabeth LANDRE, Laurence LEGRAND, Michael MANN, Jean-Louis MAS, Eric MEARY, Jean-François MEDER, Charles MELLERIO, Cristina MONDET, Olivier NAGARRA, François NATAF, Philippe PAGE, Mélanie PAGES, Vladislav PAVLOV, Thomas ROUJEAU, François-Xavier ROUX, Raphaëlle SOUILLARD-SCEMAMA, Arnault TAUZIEDE-ESPARIAT, Baris TURAK, Denis TRYSTRAM, Pascale VARLET.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Roux, A., Peeters, S., Zanello, M. et al. Extent of resection and Carmustine wafer implantation safely improve survival in patients with a newly diagnosed glioblastoma: a single center experience of the current practice. J Neurooncol 135, 83–92 (2017). https://doi.org/10.1007/s11060-017-2551-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2551-4