Abstract
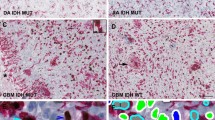
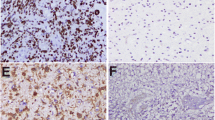
High-grade gliomas have an aggressive clinical course and new clinical biomarkers and therapeutic targets are highly needed. WEE1 is a regulator of the G2 checkpoint in glioblastoma (GBM) cells. Inhibition of this kinase has, in experimental glioma studies, been suggested to enhance sensitivity to irradiation and temozolomide. However, expression level and prognostic potential of WEE1 protein in gliomas remain uninvestigated. In this study, glioma samples from 235 patients across all four WHO grades were analyzed by immunohistochemistry. Using image analysis, we calculated the area fraction of WEE1 positive nuclei. We found that WEE1 protein was localized in tumor cell nuclei and expressed in all glioma types and grades. Although WEE1 protein levels are higher in GBMs (mean 24.5 %) relative to grade III (mean 14,0 %, p < 0.05) and grade II (mean 6.8 %, p < 0.001) gliomas, high WEE1 protein was associated with better survival in GBMs (p = 0.002). This was confirmed in multivariate analysis (HR 0.60, p = 0.003) even when adjusted for MGMT status (HR 0.60, p = 0.005). In conclusion, we report a nuclear expression of WEE1 protein in all glioma grades and types. The WEE1 positive nuclear area was correlated with malignancy grade but it was inversely associated with prognosis in GBM. Although WEE1 is a frequently occurring protein and has been proposed as a novel target in GBM, the role of WEE1 in glioma patient survival appears to be connected to the MGMT status and is more complex than previously anticipated.



Similar content being viewed by others
References
Louis DN, Ohgaki H, Wiestler OD et al (2007) WHO classification of tumours of the central nervous system, 4th edn. International Agency for Research on Cancer (IARC), Lyon
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466
Kesari S (2011) Understanding glioblastoma tumor biology: the potential to improve current diagnosis and treatments. Semin Oncol 38:S2–S10
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359:492–507
Manning G, Whyte DB, Martinez R et al (2002) The protein kinase complement of the human genome. Science 298:1912–1934
De Witt Hamer PC, Mir SE, Noske D et al (2011) WEE1 kinase targeting combined with DNA-damaging cancer therapy catalyzes mitotic catastrophe. Clin Cancer Res 17:4200–4207
Mir SE, De Witt Hamer PC, Krawczyk PM et al (2010) In silico analysis of kinase expression identifies WEE1 as a gatekeeper against mitotic catastrophe in glioblastoma. Cancer Cell 18:244–257
Wang Y, Li J, Booher RN et al (2001) Radiosensitization of p53 Mutant Cells by PD0166285, a Novel G2 Checkpoint Abrogator. Cancer Res 61:8211–8217
Iorns E, Lord CJ, Grigoriadis A et al (2009) Integrated functional, gene expression and genomic analysis for the identification of cancer targets. PLoS One 4:e5120
Masaki T, Shiratori Y, Rengifo W et al (2003) Cyclins and cyclin-dependent kinases: comparative study of hepatocellular carcinoma versus cirrhosis. Hepatology 37:534–543
Blenk S, Engelmann JC, Pinkert MWS et al (2008) Explorative data analysis of MCL reveals gene expression networks implicated in survival and prognosis supported by explorative CGH analysis. BMC Cancer 8:106
Dahlrot RH, Kristensen BW, Hjelmborg J et al (2013) A population-based study of high-grade gliomas and mutated isocitrate dehydrogenase 1. Int J Clin Exp Pathol 6:31–40
Dahlrot RH, Kristensen BW, Hjelmborg J et al (2013) A population-based study of low-grade gliomas and mutated isocitrate dehydrogenase 1 (IDH1). J Neurooncol 114:309–317
Hermansen SK, Dahlrot RH, Nielsen BS et al (2013) MiR-21 expression in the tumor cell compartment holds unfavorable prognostic value in gliomas. J Neurooncol 111(1):71–81
Dahlrot RH, Hansen S, Herrstedt J et al (2013) Prognostic value of Musashi-1 in gliomas. J Neurooncol 115:453–461
Lathia JD, Li M, Sinyuk M (2014) High-throughput flow cytometry screening reveals a role for junctional adhesion molecule a as a cancer stem cell maintenance factor. Cell Rep 6(1):117–129
Dahlrot RH, Hansen S, Jensen SS et al (2014) Clinical value of CD133 and nestin in patients with glioma: a population-based study. Int J Clin Exp Pathol 7(7):3739–3751
PosthumaDeBoer J, Wurdinger T, Graat H et al (2011) WEE1 inhibition sensitizes osteosarcoma to radiotherapy. BMC Cancer 11:156
Mueller S, Hashizume R, Yang X et al (2013) Targeting Wee1 for the treatment of pediatric high-grade gliomas. Neuro-Oncology 16(3):352–360
Harris PS, Venkataraman S, Alimova I et al (2014) Integrated genomic analysis identifies the mitotic checkpoint kinase WEE1 as a novel therapeutic target in medulloblastoma. Mol Cancer 13:1–14
Hong KS, Kim HS, Kim SH et al (2011) Hypoxia induces WEE1 expression and attenuates hydrogen peroxide-induced endothelial damage in MS1 cells. Exp Mol Med 43:653–659
Yoshida T, Tanaka S, Mogi A et al (2004) The clinical significance of cyclin B1 and Wee1 expression in non-small-cell lung cancer. Ann Oncol 15:252–256
Guertin AD, Li J, Liu Y et al (2013) Preclinical evaluation of the WEE1 inhibitor MK-1775 as single-agent anticancer therapy. Mol Cancer Ther 12:1442–1452
Kreahling JM, Gemmer JY, Reed D et al (2011) MK1775, a selective Wee1 inhibitor, shows single-agent antitumor activity against sarcoma cells. Mol Cancer Ther 11(1):174–182
Sarcar B, Kahali S, Prabhu AH et al (2011) Targeting radiation-induced G2 checkpoint activation with the Wee-1 inhibitor MK-1775 in glioblastoma cell lines. Mol Cancer Ther 10(12):2405–2414
Cancer Incidence by Age. Cancer Research UK. http://www.cancerresearchuk.org/cancer-info/cancerstats/incidence/age/. Accessed 26 Nov 2014
Do Cancer Rates Differ with Age? Australian Institute of Health and Welfare http://www.aihw.gov.au/cancer/. Accessed 26 Nov 2014
Finkel T, Serrano M, Blasco MA (2007) The common biology of cancer and ageing. Nature 448:767–774
Ohgaki H, Kleihues P (2005) Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol 64:479–489
Simon M, Hosen I, Gousias K et al (2015) TERT promoter mutations: a novel independent prognostic factor in primary glioblastomas. Neuro-Oncology 17:45–52
Hegi ME, Diserens AC, Gorlia T et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352(10):997–1003
Acknowledgments
We acknowledge the excellent laboratory work performed by technicians Helle Wohlleben and Tanja Dreehsen Højgaard.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Music, D., Dahlrot, R.H., Hermansen, S.K. et al. Expression and prognostic value of the WEE1 kinase in gliomas. J Neurooncol 127, 381–389 (2016). https://doi.org/10.1007/s11060-015-2050-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-2050-4