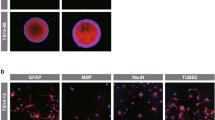
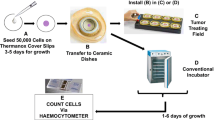
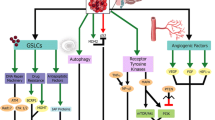
Abstract
Glioblastoma multiforme (GBM) is an aggressive, malignant cancer Johnson and O’Neill (J Neurooncol 107: 359–364, 2012). An extract from the winter cherry plant (Withania somnifera ), AshwaMAX, is concentrated (4.3 %) for Withaferin A; a steroidal lactone that inhibits cancer cells Vanden Berghe et al. (Cancer Epidemiol Biomark Prev 23: 1985–1996, 2014). We hypothesized that AshwaMAX could treat GBM and that bioluminescence imaging (BLI) could track oral therapy in orthotopic murine models of glioblastoma. Human parietal-cortical glioblastoma cells (GBM2, GBM39) were isolated from primary tumors while U87-MG was obtained commercially. GBM2 was transduced with lentiviral vectors that express Green Fluorescent Protein (GFP)/firefly luciferase fusion proteins. Mutational, expression and proliferative status of GBMs were studied. Intracranial xenografts of glioblastomas were grown in the right frontal regions of female, nude mice (n = 3–5 per experiment). Tumor growth was followed through BLI. Neurosphere cultures (U87-MG, GBM2 and GBM39) were inhibited by AshwaMAX at IC50 of 1.4, 0.19 and 0.22 µM equivalent respectively and by Withaferin A with IC50 of 0.31, 0.28 and 0.25 µM respectively. Oral gavage, every other day, of AshwaMAX (40 mg/kg per day) significantly reduced bioluminescence signal (n = 3 mice, p < 0.02, four parameter non-linear regression analysis) in preclinical models. After 30 days of treatment, bioluminescent signal increased suggesting onset of resistance. BLI signal for control, vehicle-treated mice increased and then plateaued. Bioluminescent imaging revealed diffuse growth of GBM2 xenografts. With AshwaMAX, GBM neurospheres collapsed at nanomolar concentrations. Oral treatment studies on murine models confirmed that AshwaMAX is effective against orthotopic GBM. AshwaMAX is thus a promising candidate for future clinical translation in patients with GBM.





Similar content being viewed by others
Abbreviations
- BLI:
-
Bioluminescence Imaging
- DAPI:
-
4′,6-Diamidino-2-Phenylindole
- h-EGF:
-
Human Epidermal Growth Factor
- EGFR:
-
Epidermal Growth Factor Receptor
- EGFRvIII:
-
Epidermal Growth Factor Receptor variant III
- h-FGF:
-
Human Fibroblast Growth Factor
- GBM:
-
Glioblastoma Multiforme
- GBM2:
-
Patient-derived glioblastoma multiforme cell culture (from Stanford University School of Medicine)
- GBM2/gfp/luc:
-
GBM2 that is genetically modified to express a fusion protein of firefly luciferase and Green Fluorescence Protein (GFP)
- GBM39:
-
Patient-derived glioblastoma multiforme cell culture (from University of California at San Diego School of Medicine)
- GFP:
-
Green Fluorescent Protein
- GFP/Luc:
-
Fusion protein of Green Fluorescence Protein and firefly luciferase
- HSP70:
-
Heat Shock Protein 70
- PBS:
-
Phosphate Buffered Saline
- h-PDGF-AA:
-
Human Platelet-derived Growth Factor variant AA
- h-PDGF-BB:
-
Human Platelet-derived Growth Factor variant BB
- U87:
-
GBM cell line purchased from ATCC
References
Johnson DR, O’Neill BP (2012) Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol 107:359–364. doi:10.1007/s11060-011-0749-4
Vanden Berghe W, Sabbe L, Kaileh M, Haegeman G, Heyninck K (2012) Molecular insight in the multifunctional activities of Withaferin A. Biochem Pharmacol 84:1282–1291. doi:10.1016/j.bcp.2012.08.027
Thakkar JP, Dolecek TA, Horbinski C, Ostrom QT, Lightner DD, Barnholtz-Sloan JS, Villano JL (2014) Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomark Prev 23:1985–1996. doi:10.1158/1055-9965.EPI-14-0275
Le Mercier M, Hastir D, Moles Lopez X, De Neve N, Maris C, Trepant AL, Rorive S, Decaestecker C, Salmon I (2012) A simplified approach for the molecular classification of glioblastomas. PLoS ONE 7:e45475. doi:10.1371/journal.pone.0045475
Cloughesy TF, Cavenee WK, Mischel PS (2014) Glioblastoma: from molecular pathology to targeted treatment. Annu Rev Pathol 9:1–25. doi:10.1146/annurev-pathol-011110-130324
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, O’Kelly M, Tamayo P, Weir BA, Gabriel S, Winckler W, Gupta S, Jakkula L, Feiler HS, Hodgson JG, James CD, Sarkaria JN, Brennan C, Kahn A, Spellman PT, Wilson RK, Speed TP, Gray JW, Meyerson M, Getz G, Perou CM, Hayes DN (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17:98–110. doi:10.1016/j.ccr.2009.12.020
Zhang X, Zhang W, Cao WD, Cheng G, Zhang YQ (2012) Glioblastoma multiforme: molecular characterization and current treatment strategy (Review). Exp Ther Med 3:9–14. doi:10.3892/etm.2011.367
Olar A, Aldape KD (2014) Using the molecular classification of glioblastoma to inform personalized treatment. J Pathol 232:165–177. doi:10.1002/path.4282
Frederick L, Wang XY, Eley G, James CD (2000) Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res 60:1383–1387
Grasso CS, Tang Y, Truffaux N, Berlow NE, Liu L, Debily MA, Quist MJ, Davis LE, Huang EC, Woo PJ, Ponnuswami A, Chen S, Johung TB, Sun W, Kogiso M, Du Y, Qi L, Huang Y, Hutt-Cabezas M, Warren KE, Le Dret L, Meltzer PS, Mao H, Quezado M, van Vuurden DG, Abraham J, Fouladi M, Svalina MN, Wang N, Hawkins C, Nazarian J, Alonso MM, Raabe EH, Hulleman E, Spellman PT, Li XN, Keller C, Pal R, Grill J, Monje M (2015) Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nat Med. doi:10.1038/nm.3855
Mishra LC, Singh BB, Dagenais S (2000) Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Alternative Med Rev 5:334–346
Weir SJ, DeGennaro LJ, Austin CP (2012) Repurposing approved and abandoned drugs for the treatment and prevention of cancer through public-private partnership. Cancer Res 72:1055–1058. doi:10.1158/0008-5472.CAN-11-3439
Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP (2014) Repurposing drugs in oncology (ReDO)-mebendazole as an anti-cancer agent. Ecancermedicalscience 8:443. doi:10.3332/ecancer.2014.443
Lavie D, Glotter E, Shvo Y (1965) Constituents of Withania somnifera Dun. III. The side chain of Withaferin A. J Org Chem 30:1774–1778
Mirjalili MH, Moyano E, Bonfill M, Cusido RM, Palazon J (2009) Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules 14:2373–2393. doi:10.3390/molecules14072373
Prasanna KS, Shilpa P, Salimath BP (2009) Withaferin A suppresses the expression of vascular endothelial growth factor in Ehrlich ascites tumor cells via Sp1 transcription factor. Curr Trends Biotechnol Pharm 3:138–148
Heyninck K, Lahtela-Kakkonen M, Van der Veken P, Haegeman G, Vanden Berghe W (2014) Withaferin A inhibits NF-kappaB activation by targeting cysteine 179 in IKKbeta. Biochem Pharmacol 91:501–509. doi:10.1016/j.bcp.2014.08.004
Khan S, Rammeloo AW, Heikkila JJ (2012) Withaferin A induces proteasome inhibition, endoplasmic reticulum stress, the heat shock response and acquisition of thermotolerance. PLoS ONE 7:e50547. doi:10.1371/journal.pone.0050547
Jilani K, Lupescu A, Zbidah M, Shaik N, Lang F (2013) Withaferin A-stimulated Ca2+ entry, ceramide formation and suicidal death of erythrocytes. Toxicol Vitro 27:52–58. doi:10.1016/j.tiv.2012.09.004
Sharada AC, Solomon FE, Devi PU, Udupa N, Srinivasan KK (1996) Antitumor and radiosensitizing effects of withaferin A on mouse Ehrlich ascites carcinoma in vivo. Acta Oncol 35:95–100
Devi PU, Sharada AC, Solomon FE (1993) Antitumor and radiosensitizing effects of Withania somnifera (Ashwagandha) on a transplantable mouse tumor, Sarcoma-180. Indian J Exp Biol 31:607–611
Widodo N, Kaur K, Shrestha BG, Takagi Y, Ishii T, Wadhwa R, Kaul SC (2007) Selective killing of cancer cells by leaf extract of Ashwagandha: identification of a tumor-inhibitory factor and the first molecular insights to its effect. Clin Cancer Res 13:2298–2306. doi:10.1158/1078-0432.CCR-06-0948
Shohat B, Gitter S, Lavie D (1970) Effect of withaferin A on Ehrlich ascites tumor cells–cytological observations. Int J Cancer 5:244–252
Shohat B, Gitter S, Abraham A, Lavie D (1967) Antitumor activity of withaferin A (NSC-101088). Cancer Chemother Rep Part 1(51):271–276
Patel S, Rao NHL (2014) Safety assessment of Withania Somnifera extract standardized for Withaferin A: acute and Sub-acute toxicity study
Sarkaria JN, Yang L, Grogan PT, Kitange GJ, Carlson BL, Schroeder MA, Galanis E, Giannini C, Wu W, Dinca EB, James CD (2007) Identification of molecular characteristics correlated with glioblastoma sensitivity to EGFR kinase inhibition through use of an intracranial xenograft test panel. Mol Cancer Ther 6:1167–1174. doi:10.1158/1535-7163.MCT-06-0691
Sasportas LS, Gambhir SS (2014) Imaging circulating tumor cells in freely moving awake small animals using a miniaturized intravital microscope. PLoS ONE 9:e86759. doi:10.1371/journal.pone.0086759
Anoopkumar-Dukie S, Carey JB, Conere T, O’Sullivan E, van Pelt FN, Allshire A (2005) Resazurin assay of radiation response in cultured cells. Br J Radiol 78:945–947. doi:10.1259/bjr/54004230
Lecoeur H (2002) Nuclear apoptosis detection by flow cytometry: influence of endogenous endonucleases. Exp Cell Res 277:1–14. doi:10.1006/excr.2002.5537
Yoshimoto K, Dang J, Zhu S, Nathanson D, Huang T, Dumont R, Seligson DB, Yong WH, Xiong Z, Rao N, Winther H, Chakravarti A, Bigner DD, Mellinghoff IK, Horvath S, Cavenee WK, Cloughesy TF, Mischel PS (2008) Development of a real-time RT-PCR assay for detecting EGFRvIII in glioblastoma samples. Clin Cancer Res 14:488–493. doi:10.1158/1078-0432.CCR-07-1966
Baumann BC, Dorsey JF, Benci JL, Joh DY, Kao GD (2012) Stereotactic intracranial implantation and in vivo bioluminescent imaging of tumor xenografts in a mouse model system of glioblastoma multiforme. J Vis Exp. doi:10.3791/4089
Bergamino M, Bonzano L, Levrero F, Mancardi GL, Roccatagliata L (2014) A review of technical aspects of T1-weighted dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) in human brain tumors. Phys Med 30:635–643. doi:10.1016/j.ejmp.2014.04.005
Llewellyn BD (2009) Nuclear staining with alum hematoxylin. Biotech Histochem 84:159–177. doi:10.1080/10520290903052899
Del Vecchio CA, Giacomini CP, Vogel H, Jensen KC, Florio T, Merlo A, Pollack JR, Wong AJ (2013) EGFRvIII gene rearrangement is an early event in glioblastoma tumorigenesis and expression defines a hierarchy modulated by epigenetic mechanisms. Oncogene 32:2670–2681. doi:10.1038/onc.2012.280
Sepulveda JM, Belda-Iniesta C, Gil-Gil M, Perez-Segura P, Berrocal A, Reynes G, Gallego O, Capellades J, Ordonez JM, La Orden B, Balana C (2015) A phase II study of feasibility and toxicity of bevacizumab in combination with temozolomide in patients with recurrent glioblastoma. Clin Transl Oncol. doi:10.1007/s12094-015-1304-0
Johansson F, Ekman S, Blomquist E, Henriksson R, Bergstrom S, Bergqvist M (2012) A review of dose-dense temozolomide alone and in combination with bevacizumab in patients with first relapse of glioblastoma. Anticancer Res 32:4001–4006
Joh DY, Sun L, Stangl M, Al Zaki A, Murty S, Santoiemma PP, Davis JJ, Baumann BC, Alonso-Basanta M, Bhang D, Kao GD, Tsourkas A, Dorsey JF (2013) Selective targeting of brain tumors with gold nanoparticle-induced radiosensitization. PLoS ONE 8:e62425. doi:10.1371/journal.pone.0062425
Coluccia D, Fandino J, Schwyzer L, O’Gorman R, Remonda L, Anon J, Martin E, Werner B (2014) First noninvasive thermal ablation of a brain tumor with MR-guided focused ultrasound. J Ther Ultrasound 2:17. doi:10.1186/2050-5736-2-17
Raut AA, Rege NN, Tadvi FM, Solanki PV, Kene KR, Shirolkar SG, Pandey SN, Vaidya RA, Vaidya AB (2012) Exploratory study to evaluate tolerability, safety, and activity of Ashwagandha (Withania somnifera) in healthy volunteers. J Ayurveda Integr Med 3:111–114. doi:10.4103/0975-9476.100168
Wadhwa R, Singh R, Gao R, Shah N, Widodo N, Nakamoto T, Ishida Y, Terao K, Kaul SC (2013) Water extract of Ashwagandha leaves has anticancer activity: identification of an active component and its mechanism of action. PLoS ONE 8:e77189. doi:10.1371/journal.pone.0077189
Sandhu JS, Shah B, Shenoy S, Chauhan S, Lavekar GS, Padhi MM (2010) Effects of Withania somnifera (Ashwagandha) and Terminalia arjuna (Arjuna) on physical performance and cardiorespiratory endurance in healthy young adults. Int J Ayurveda Res 1:144–149. doi:10.4103/0974-7788.72485
Satelli A, Li S (2011) Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci 68:3033–3046. doi:10.1007/s00018-011-0735-1
Bargagna-Mohan P, Hamza A, Kim YE, Khuan Abby Ho Y, Mor-Vaknin N, Wendschlag N, Liu J, Evans RM, Markovitz DM, Zhan CG, Kim KB, Mohan R (2007) The tumor inhibitor and antiangiogenic agent withaferin A targets the intermediate filament protein vimentin. Chem Biol 14:623–634. doi:10.1016/j.chembiol.2007.04.010
Yang H, Shi G, Dou QP (2007) The tumor proteasome is a primary target for the natural anticancer compound Withaferin A isolated from “Indian winter cherry”. Mol Pharmacol 71:426–437. doi:10.1124/mol.106.030015
Grover A, Shandilya A, Punetha A, Bisaria VS, Sundar D (2010) Inhibition of the NEMO/IKKbeta association complex formation, a novel mechanism associated with the NF-kappaB activation suppression by Withania somnifera’s key metabolite withaferin A. BMC Genom 11(Suppl 4):S25. doi:10.1186/1471-2164-11-S4-S25
Yu Y, Hamza A, Zhang T, Gu M, Zou P, Newman B, Li Y, Gunatilaka AA, Zhan CG, Sun D (2010) Withaferin A targets heat shock protein 90 in pancreatic cancer cells. Biochem Pharmacol 79:542–551. doi:10.1016/j.bcp.2009.09.017
Grover A, Shandilya A, Agrawal V, Pratik P, Bhasme D, Bisaria VS, Sundar D (2011) Hsp90/Cdc37 chaperone/co-chaperone complex, a novel junction anticancer target elucidated by the mode of action of herbal drug Withaferin A. BMC Bioinformatics 12(Suppl 1):S30. doi:10.1186/1471-2105-12-S1-S30
Sen N, Banerjee B, Das BB, Ganguly A, Sen T, Pramanik S, Mukhopadhyay S, Majumder HK (2007) Apoptosis is induced in leishmanial cells by a novel protein kinase inhibitor withaferin A and is facilitated by apoptotic topoisomerase I-DNA complex. Cell Death Differ 14:358–367. doi:10.1038/sj.cdd.4402002
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J (2015) Structural and functional features of central nervous system lymphatic vessels. Nature. doi:10.1038/nature14432
Acknowledgments
GBM39 was the donated to us by Dr. Paul Mischel, MD, PhD while the cell lines of GBM2 and GBM2/gfp/Luc were kindly provided to us by Dr. Michelle Monje, MD, PhD. We thank Dr’s. Laura Pisani, PhD and Timothy Doyle, PhD of the Stanford Small Animal Imaging Facility (SAIF) for their excellent technical support. AshwaMAX was obtained from Dr. Lal Hingorani from Pharmaza Herbal Pvt Ltd. (Gujarat, India). We acknowledge the excellent technical support of Kathryn Li, Tara Thakurta, Alex Serafini and Xiaofan Wu.
Funding
We gratefully acknowledge the Ben and Catherine Ivy Foundation for funding of the research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The Authors declare no conflict of interests
Animal studies
All preclinical studies were approved by the Institutional Animal Care and Use Committee of Stanford University. All ethical guidelines and standards were adhered to.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chang, E., Pohling, C., Natarajan, A. et al. AshwaMAX and Withaferin A inhibits gliomas in cellular and murine orthotopic models. J Neurooncol 126, 253–264 (2016). https://doi.org/10.1007/s11060-015-1972-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1972-1