Abstract
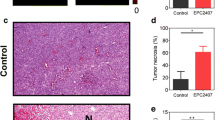
Small animal imaging is of increasing relevance in biomedical research. Studies systematically assessing the diagnostic accuracy of contrast-enhanced in vivo micro-CT of orthotopic glioma xenografts in mice do not exist. NOD/SCID/γc−/− mice (n = 27) underwent intracerebral implantation of 2.5 × 106 GFP-Luciferase-transduced U87MG cells. Mice underwent bioluminescence imaging (BLI) to detect tumor growth and afterwards repeated contrast-enhanced (300 µl Iomeprol i.v.) micro-CT imaging (80 kV, 75 µAs, 360° rotation, 1,000 projections, 33 s scan time, resolution 40 × 40 × 53 µm, 0.5 Gy/scan). Presence of tumors, tumor diameter and tumor volume in micro-CT were rated by two independent readers. Results were compared with histological analyses. Six mice with tumors confirmed by micro-CT received fractionated irradiation (3 × 5 Gy every other day) using the micro-CT (5 mm pencil beam geometry). Repeated micro-CT scans were tolerated well. Tumor engraftment rate was 74 % (n = 20). In micro-CT, mean tumor volume was 30 ± 33 mm3, and the smallest detectable tumor measured 360 × 620 µm. The inter-rater agreement (n = 51 micro-CT scans) for the item tumor yes/no was excellent (Spearman-Rho = 0.862, p < 0.001). Sensitivity and specificity of micro-CT were 0.95 and 0.71, respectively (PPV = 0.91, NPV = 0.83). BLI on day 21 after tumor implantation had a sensitivity and specificity of 0.90 and 1.0, respectively (PPV = 1.0, NPV = 0.5). Maximum tumor diameter and volume in micro-CT and histology correlated excellently (tumor diameter: 0.929, p < 0.001; tumor volume: 0.969, p < 0.001, n = 17). Irradiated animals showed a large central tumor necrosis. Longitudinal contrast enhanced micro-CT imaging of brain tumor growth in live mice is feasible at high sensitivity levels and with excellent inter-rater agreement and allows visualization of radiation effects.




Similar content being viewed by others
References
Kang KB, Zhu C, Wong YL, Gao Q, Ty A, Wong MC (2012) Gefitinib radiosensitizes stem-like glioma cells: inhibition of epidermal growth factor receptor-Akt-DNA-PK signaling, accompanied by inhibition of DNA double-strand break repair. Int J Radiat Oncol Biol Phys 83(1):e43–e52
Lobo MR, Green SC, Schabel MC, Gillespie GY, Woltjer RL, Pike MM (2013) Quinacrine synergistically enhances the antivascular and antitumor efficacy of cediranib in intracranial mouse glioma. Neuro Oncol 15(12):1673–1683
Brockmann MA, Kemmling A, Groden C (2007) Current issues and perspectives in small rodent magnetic resonance imaging using clinical MRI scanners. Methods 43(1):79–87
Brockmann MA, Ulmer S, Leppert J et al (2006) Analysis of mouse brain using a clinical 1.5 T scanner and a standard small loop surface coil. Brain Res 1068(1):138–142
Engelhorn T, Eyupoglu IY, Schwarz MA et al (2009) In vivo micro-CT imaging of rat brain glioma: a comparison with 3T MRI and histology. Neurosci Lett 458(1):28–31
Schwarz M, Engelhorn T, Eyupoglu IY et al (2010) In vivo imaging of MSCT and micro-CT: a comparison. Rofo 182(4):322–326
Menk RH, Schultke E, Hall C et al (2011) Gold nanoparticle labeling of cells is a sensitive method to investigate cell distribution and migration in animal models of human disease. Nanomedicine 7(5):647–654
Menichetti L, Petroni D, Panetta D et al (2011) A micro-PET/CT approach using O-(2-[18F]fluoroethyl)-l-tyrosine in an experimental animal model of F98 glioma for BNCT. Appl Radiat Isot 69(12):1717–1720
Park SS, Chunta JL, Robertson JM et al (2011) MicroPET/CT imaging of an orthotopic model of human glioblastoma multiforme and evaluation of pulsed low-dose irradiation. Int J Radiat Oncol Biol Phys 80(3):885–892
Lee DY, Chunta JL, Park SS et al (2013) Pulsed versus conventional radiation therapy in combination with temozolomide in a murine orthotopic model of glioblastoma multiforme. Int J Radiat Oncol Biol Phys 86(5):978–985
Dilworth JT, Krueger SA, Dabjan M et al (2013) Pulsed low-dose irradiation of orthotopic glioblastoma multiforme (GBM) in a pre-clinical model: effects on vascularization and tumor control. Radiother Oncol 108(1):149–154
Kepshire DS, Gibbs-Strauss SL, O’Hara JA et al (2009) Imaging of glioma tumor with endogenous fluorescence tomography. J biomed Optics 14(3):030501
Maier P, Herskind C, Barzan D, Zeller WJ, Wenz F (2010) SNAI2 as a novel radioprotector of normal tissue by gene transfer using a lentiviral bicistronic SIN vector. Radiat Res 173(5):612–619
Demaison C, Parsley K, Brouns G et al (2002) High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency [correction of imunodeficiency] virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Human Gene Ther 13(7):803–813
Schambach SJ, Bag S, Groden C, Schilling L, Brockmann MA (2010) Vascular imaging in small rodents using micro-CT. Methods 50(1):26–35
Schambach SJ, Bag S, Schilling L, Groden C, Brockmann MA (2010) Application of micro-CT in small animal imaging. Methods 50(1):2–13
Figueiredo G, Brockmann C, Boll H et al (2012) Comparison of digital subtraction angiography, micro-computed tomography angiography and magnetic resonance angiography in the assessment of the cerebrovascular system in live mice. Clin Neuroradiol 22(1):21–28
Boll H, Figueiredo G, Fiebig T et al (2013) Comparison of Fenestra LC, ExiTron nano 6000, and ExiTron nano 12000 for micro-CT imaging of liver and spleen in mice. Acad Radiol 20(9):1137–1143
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28(11):1963–1972
Ma C-M et al (2001) AAPM protocol for 40–300 kV x-ray beam dosimetry in radiotherapy ans radiobiology. Med Phys 28(6):868–893
Schmidt KF, Ziu M, Schmidt NO et al (2004) Volume reconstruction techniques improve the correlation between histological and in vivo tumor volume measurements in mouse models of human gliomas. J Neurooncol 68(3):207–215
Saito S, Murase K (2012) Ex vivo imaging of mouse brain using micro-CT with non-ionic iodinated contrast agent: a comparison with myelin staining. Br J Radiol 85(1019):e973–e978
de Crespigny A, Bou-Reslan H, Nishimura MC, Phillips H, Carano RA, D’Arceuil HE (2008) 3D micro-CT imaging of the postmortem brain. J Neurosci Methods 171(2):207–213
Huang M, Xiong C, Lu W et al (2014) Dual-modality micro-positron emission tomography/computed tomography and near-infrared fluorescence imaging of EphB4 in orthotopic glioblastoma xenograft models. Mol Imag Biol 16(1):74–84
Newcomb EW, Demaria S, Lukyanov Y et al (2006) The combination of ionizing radiation and peripheral vaccination produces long-term survival of mice bearing established invasive GL261 gliomas. Clin Cancer Res 12(15):4730–4737
Yahyanejad S, Granton PV, Lieuwes NG et al (2014) Complementary use of bioluminescence imaging and contrast-enhanced micro-computed tomography in an orthotopic brain tumor model. Mol Imag 13:1–8
Jost SC, Collins L, Travers S, Piwnica-Worms D, Garbow JR (2009) Measuring brain tumor growth: combined bioluminescence imaging-magnetic resonance imaging strategy. Mol Imag 8(5):245–253
Balvay D, Tropres I, Billet R et al (2009) Mapping the zonal organization of tumor perfusion and permeability in a rat glioma model by using dynamic contrast-enhanced synchrotron radiation CT. Radiology 250(3):692–702
Figueiredo G, Boll H, Kramer M, Groden C, Brockmann MA (2012) In vivo X-ray digital subtraction and CT angiography of the murine cerebrovasculature using an intra-arterial route of contrast injection. AJNR Am J Neuroradiol 33(9):1702–1709
Momota H, Holland EC (2009) Mouse models of CNS embryonal tumors. Brain Tumor Pathol 26(2):43–50
Shih AH, Dai C, Hu X, Rosenblum MK, Koutcher JA, Holland EC (2004) Dose-dependent effects of platelet-derived growth factor-B on glial tumorigenesis. Cancer Res 64(14):4783–4789
Kunkel P, Ulbricht U, Bohlen P et al (2001) Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res 61(18):6624–6628
Acknowledgments
We are indebted to Dr. M. Müller for assistance in preparation of this manuscript.
Conflict of interest
None of the authors of the above manuscript have declared any conflict of interest.
Funding
The acquisition of the micro-CT (Yxlon Y. Fox) was funded by the Federal Ministry of Education and Research and the Land Baden-Württemberg (HBFG Grant#125-648). This work was funded by the Deutsche Forschungsgemeinschaft (Grants FL 880/1-1, GI 771/1-1. GL 236/9-1 and WE 2063/9-1), the Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research, grant BMBF 01EZ1130), the Klaus Tschira Stiftung (Grant 00.211.2012) and the Bundesamt für Strahlenschutz (Federal Office for Radiation Protection, Grant BfS 3608S04001) for the establishment of the endowed professorship Medizinische Strahlenphysik/Strahlenschutz (Medical Radiation Physics/Radiation Protection).
Author information
Authors and Affiliations
Corresponding author
Additional information
Frank A. Giordano and Marc A. Brockmann these authors have contributed equally.
Rights and permissions
About this article
Cite this article
Kirschner, S., Felix, M.C., Hartmann, L. et al. In vivo micro-CT imaging of untreated and irradiated orthotopic glioblastoma xenografts in mice: capabilities, limitations and a comparison with bioluminescence imaging. J Neurooncol 122, 245–254 (2015). https://doi.org/10.1007/s11060-014-1708-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1708-7