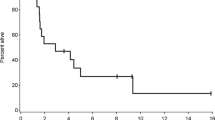
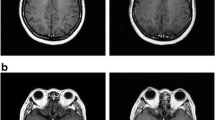
Brain metastases (BM) and leptomeningeal metastases (LM) are devastating neurologic complications. Pemetrexed is a multi-targeted anti-folate agent approved for treatment of nonsquamous non-small cell lung cancer but has anti-tumor activity in other solid tumors. We performed two trials using pemetrexed in patients with BM and LM to assess CSF penetration and anti-tumor activity. Patients were treated with intravenous pemetrexed at doses of 500 (n = 3), 750 (n = 3), 900 (n = 12) or 1,050 mg/m2 (n = 3) every 3 weeks. Neuro-imaging was done every 6 weeks. Matched CSF and plasma samples were obtained serially from three patients with Ommaya reservoirs; the remaining patients had a single paired collection. Twenty-one patients (15 women and six men) with median age of 50 years and median KPS of 90 were treated. Primary tumors included breast (13), lung (4), colorectal (1), endometrial (1), esophageal (1) and pinealoblastoma (1). Nine patients had prior whole brain RT and median number of prior chemotherapies was two including prior methotrexate in four patients. Median pemetrexed doses administered was three (range 1–14). Responses included one partial response, ten stable disease and ten progressive disease. Median time to progression and survival was 2.7 and 7.3 months; PFS six was 22 %. No major toxicities were seen. Pemetrexed distributed from the plasma to the CSF within 1–4 h with the resulting CSF concentrations < 5 % of plasma. Pemetrexed was tolerated in solid tumor patients with CNS metastases. Limited anti-tumor activity was seen, which might have been due to low CSF concentrations, although some patients displayed prolonged benefit.




Similar content being viewed by others
References
Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, Jain RK (2011) The biology of brain metastases-translation to new therapies. Nat Rev Clin oncol 8(6):344–356
Chamberlain MC (2009) Leptomeningeal metastasis. Curr Opin Neurol 22(6):665–674
Yamanaka R (2009) Medical management of brain metastases from lung cancer (Review). Oncol Rep 22(6):1269–1276
Cheng X, Hung MC (2007) Breast cancer brain metastases. Cancer Metastasis Rev 26(3–4):635–643
Gaspar LE, Mehta MP, Patchell RA, Burri SH, Robinson PD, Morris RE, Ammirati M, Andrews DW, Asher AL, Cobbs CS, Kondziolka D, Linskey ME, Loeffler JS, McDermott M, Mikkelsen T, Olson JJ, Paleologos NA, Ryken TC, Kalkanis SN (2010) The role of whole brain radiation therapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 96(1):17–32
Linskey ME, Andrews DW, Asher AL, Burri SH, Kondziolka D, Robinson PD, Ammirati M, Cobbs CS, Gaspar LE, Loeffler JS, McDermott M, Mehta MP, Mikkelsen T, Olson JJ, Paleologos NA, Patchell RA, Ryken TC, Kalkanis SN (2010) The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 96(1):45–68
Ammirati M, Cobbs CS, Linskey ME, Paleologos NA, Ryken TC, Burri SH, Asher AL, Loeffler JS, Robinson PD, Andrews DW, Gaspar LE, Kondziolka D, McDermott M, Mehta MP, Mikkelsen T, Olson JJ, Patchell RA, Kalkanis SN (2010) The role of retreatment in the management of recurrent/progressive brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 96(1):85–96
Lassman AB, Abrey LE, Shah GD, Panageas KS, Begemann M, Malkin MG, Raizer JJ (2006) Systemic high-dose intravenous methotrexate for central nervous system metastases. J Neurooncol 78(3):255–260
Glantz MJ, Cole BF, Recht L, Akerley W, Mills P, Saris S, Hochberg F, Calabresi P, Egorin MJ (1998) High-dose intravenous methotrexate for patients with nonleukemic leptomeningeal cancer: is intrathecal chemotherapy necessary? J clin 16(4):1561–1567
Abrey LE, Christodoulou C (2001) Temozolomide for treating brain metastases. Semin Oncol 28(4 Suppl 13):34–42
Christodoulou C, Bafaloukos D, Kosmidis P, Samantas E, Bamias A, Papakostas P, Karabelis A, Bacoyiannis C, Skarlos DV (2001) Phase II study of temozolomide in heavily pretreated cancer patients with brain metastases. Ann oncol 12(2):249–254
Abrey LE, Olson JD, Raizer JJ, Mack M, Rodavitch A, Boutros DY, Malkin MG (2001) A phase II trial of temozolomide for patients with recurrent or progressive brain metastases. J Neurooncol 53(3):259–265
Lin NU, Eierman W, Greil R, Campone M, Kaufman B, Steplewski K, Lane SR, Zembryki D, Rubin SD, Winer EP (2011) Randomized phase II study of lapatinib plus capecitabine or lapatinib plus topotecan for patients with HER2-positive breast cancer brain metastases. J Neurooncol 105(3):613–620
Oberhoff C, Kieback DG, Wurstlein R, Deertz H, Sehouli J, van Soest C, Hilfrich J, Mesrogli M, von Minckwitz G, Staab HJ, Schindler AE (2001) Topotecan chemotherapy in patients with breast cancer and brain metastases: results of a pilot study. Onkologie 24(3):256–260
Lorusso V, Galetta D, Giotta F, Rinaldi A, Romito S, Brunetti C, Silvestris N, Colucci G (2006) Topotecan in the treatment of brain metastases. A phase II study of GOIM (Gruppo Oncologico dell’Italia Meridionale). Anticancer Res 26(3B):2259–2263
Tsimberidou AM, Letourneau K, Wen S, Wheler J, Hong D, Naing A, Iskander NG, Uehara C, Kurzrock R (2011) Phase I clinical trial outcomes in 93 patients with brain metastases: the MD anderson cancer center experience. Clin Cancer Res 17(12):4110–4118
Groves MD, Glantz MJ, Chamberlain MC, Baumgartner KE, Conrad CA, Hsu S, Wefel JS, Gilbert MR, Ictech S, Hunter KU, Forman AD, Puduvalli VK, Colman H, Hess KR, Yung WK (2008) A multicenter phase II trial of intrathecal topotecan in patients with meningeal malignancies. Neuro-oncology 10(2):208–215
Jaeckle KA, Phuphanich S, Bent MJ, Aiken R, Batchelor T, Campbell T, Fulton D, Gilbert M, Heros D, Rogers L, O’Day SJ, Akerley W, Allen J, Baidas S, Gertler SZ, Greenberg HS, LaFollette S, Lesser G, Mason W, Recht L, Wong E, Chamberlain MC, Cohn A, Glantz MJ, Gutheil JC, Maria B, Moots P, New P, Russell C, Shapiro W, Swinnen L, Howell SB (2001) Intrathecal treatment of neoplastic meningitis due to breast cancer with a slow-release formulation of cytarabine. Br J Cancer 84(2):157–163
Glantz MJ, Jaeckle KA, Chamberlain MC, Phuphanich S, Recht L, Swinnen LJ, Maria B, LaFollette S, Schumann GB, Cole BF, Howell SB (1999) A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res 5(11):3394–3402
Livingston RB (1986) Current chemotherapy of small cell lung cancer. Chest 89(4 Suppl):258S–263S
Samonis G, Bafaloukos D, Margioris AN, Katsarma G, Toloudis P, Bacoyannis C, Karvounis N, Georgoulias V, Kosmidis P (1997) First-line chemotherapy of advanced breast cancer with a combination of mitoxantrone, methotrexate, and vincristine (MIMO). Oncology 54(5):371–375
Clatot F, Philippin-Lauridant G, Ouvrier MJ, Nakry T, Laberge-Le-Couteulx S, Guillemet C, Veyret C, Blot E (2009) Clinical improvement and survival in breast cancer leptomeningeal metastasis correlate with the cytologic response to intrathecal chemotherapy. J Neurooncol 95(3):421–426
Kimelberg HK, Biddlecome SM, Bourke RS (1977) Distribution and degradation of [3H]methotrexate after intravenous and cerebral intraventricular injection in primates. Cancer Res 37(1):157–165
Kwong YL, Yeung DY, Chan JC (2009) Intrathecal chemotherapy for hematologic malignancies: drugs and toxicities. Ann Hematol 88(3):193–201
Reni M, Ferreri AJ, Guha-Thakurta N, Blay JY, Dell’Oro S, Biron P, Hochberg FH (2001) Clinical relevance of consolidation radiotherapy and other main therapeutic issues in primary central nervous system lymphomas treated with upfront high-dose methotrexate. Int J Radiat Oncol Biol Phys 51(2):419–425
Villela LR, Stanford BL, Shah SR (2006) Pemetrexed, a novel antifolate therapeutic alternative for cancer chemotherapy. Pharmacotherapy 26(5):641–654
Curtin NJ, Hughes AN (2001) Pemetrexed disodium, a novel antifolate with multiple targets. Lancet Oncol 2(5):298–306
Schneeweiss A, Marme F, Ruiz A, Manikhas AG, Bottini A, Wolf M, Sinn HP, Mansouri K, Kennedy L, Bauknecht T (2011) A randomized phase II trial of doxorubicin plus pemetrexed followed by docetaxel versus doxorubicin plus cyclophosphamide followed by docetaxel as neoadjuvant treatment of early breast cancer. Ann Oncol 22(3):609–617
Garin A, Manikhas A, Biakhov M, Chezhin M, Ivanchenko T, Krejcy K, Karaseva V, Tjulandin S (2008) A phase II study of pemetrexed and carboplatin in patients with locally advanced or metastatic breast cancer. Breast Cancer Res Treat 110(2):309–315
Robert NJ, Conkling PR, O’Rourke MA, Kuefler PR, McIntyre KJ, Zhan F, Asmar L, Wang Y, Shonukan OO, O’Shaughnessy JA (2011) Results of a phase II study of pemetrexed as first-line chemotherapy in patients with advanced or metastatic breast cancer. Breast Cancer Res Treat 126(1):101–108
Martin M, Blasinska-Morawiec M, Salas JF, Falcon S, Rolski J, Ferrari BL, Gulyas S, Liu Y, Benhadji KA (2009) A multicenter, single-arm phase II study of pemetrexed plus doxorubicin administered every 21 days in patients with advanced breast cancer. Clinical breast cancer 9(3):155–160
Miles DW, Smith IE, Coleman RE, Calvert AH, Lind MJ (2001) A phase II study of pemetrexed disodium (LY231514) in patients with locally recurrent or metastatic breast cancer. Eur J Cancer 37(11):1366–1371
Cohen MH, Cortazar P, Justice R, Pazdur R (2010) Approval summary: pemetrexed maintenance therapy of advanced/metastatic nonsquamous, non-small cell lung cancer (NSCLC). Oncologist 15(12):1352–1358
Bearz A, Garassino I, Tiseo M, Caffo O, Soto-Parra H, Boccalon M, Talamini R, Santoro A, Bartolotti M, Murgia V, Berretta M, Tirelli U (2010) Activity of pemetrexed on brain metastases from non-small cell lung cancer. Lung Cancer 68(2):264–268
Omlin A, D’Addario G, Gillessen S, Cerny T, von Hessling A, Fruh M (2009) Activity of pemetrexed against brain metastases in a patient with adenocarcinoma of the lung. Lung Cancer 65(3):383–384
Stapleton SL, Reid JM, Thompson PA, Ames MM, McGovern RM, McGuffey L, Nuchtern J, Dauser R, Blaney SM (2007) Plasma and cerebrospinal fluid pharmacokinetics of pemetrexed after intravenous administration in non-human primates. Cancer Chemother Pharmacol 59(4):461–466
Raizer JJ, Rademaker A, Evens AM, Rice L, Schwartz M, Chandler JP, Getch CC, Tellez C, Grimm SA (2012) Pemetrexed in the treatment of relapsed/refractory primary central nervous system lymphoma. Cancer 118(15):3743–3748
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8(7):1277–1280
Latz JE, Chaudhary A, Ghosh A, Johnson RD (2006) Population pharmacokinetic analysis of ten phase II clinical trials of pemetrexed in cancer patients. Cancer Chemother Pharmacol 57(4):401–411
Vezmar S, Becker A, Bode U, Jaehde U (2003) Biochemical and clinical aspects of methotrexate neurotoxicity. Chemotherapy 49(1–2):92–104
Sweeney CJ, Takimoto CH, Latz JE, Baker SD, Murry DJ, Krull JH, Fife K, Battiato L, Cleverly A, Chaudhary AK, Chaudhuri T, Sandler A, Mita AC, Rowinsky EK (2006) Two drug interaction studies evaluating the pharmacokinetics and toxicity of pemetrexed when coadministered with aspirin or Ibuprofen in patients with advanced cancer. Clin Cancer Res 12(2):536–542
Dickgreber NJ, Sorensen JB, Paz-Ares LG, Schytte TK, Latz JE, Schneck KB, Yuan Z, Sanchez-Torres JM (2010) Pemetrexed safety and pharmacokinetics in patients with third-space fluid. Clin Cancer Res 16(10):2872–2880
Niyikiza C, Baker SD, Seitz DE, Walling JM, Nelson K, Rusthoven JJ, Stabler SP, Paoletti P, Calvert AH, Allen RH (2002) Homocysteine and methylmalonic acid: markers to predict and avoid toxicity from pemetrexed therapy. Mol Cancer Ther 1(7):545–552
Chattopadhyay S, Tamari R, Min SH, Zhao R, Tsai E, Goldman ID (2007) Commentary: a case for minimizing folate supplementation in clinical regimens with pemetrexed based on the marked sensitivity of the drug to folate availability. Oncologist 12(7):808–815
Acknowledgments
Study was supported by Lilly Oncology (research support for JJR).
Conflict of interest
None for Priya Kumthekar, Sean A. Grimm, Michael J. Avram, Virginia Kaklamani, Irene Helenowski, Alfred Rademaker, Mary Cianfrocca, William Gradishar, Jyoti Patel, Mary Mulcahy, Katie McCarthy; Jeffrey J. Raizer has research funding from Lilly Oncology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumthekar, P., Grimm, S.A., Avram, M.J. et al. Pharmacokinetics and efficacy of pemetrexed in patients with brain or leptomeningeal metastases. J Neurooncol 112, 247–255 (2013). https://doi.org/10.1007/s11060-013-1055-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-013-1055-0