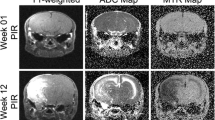
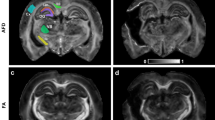
Abstract
We aim to study radiation induced white matter injury in a pre-clinical model using Diffusion tensor MR imaging (DTI). Nineteen 12-week old Sprague–Dawley rats were irradiated to the right hemisphere using a linear accelerator. The dose distribution map was coregistered to the DTI map to generate the actual radiation dose to each white matter tract. Rats underwent longitudinal DTI scans at five time points from 4 to 48 weeks post-radiation with histological evaluations. Fractional anisotropy (FA) of the external capsule, fornix, cerebral peduncle, anterior commissure, optic tract and optic nerve was evaluated. Radiation dose was highest at the ipsilateral external capsule and fornix (29.4 ± 1.3 and 29.8 ± 1.1 Gy, respectively). Optic nerve received 50 % dose to the external capsule and other white matter tracts received 80 % dose. Significantly lower FA was firstly found in the ipsilateral external capsule at 4 weeks post-radiation and in the ipsilateral fornix at 40 weeks post-radiation compared to the contralateral side. Significantly lower FA was found in contralateral optic nerve compared to ipsilateral optic nerve at 48 weeks post-radiation despite ipsilateral optic nerves receiving higher radiation dose than contralateral optic nerve (p = 0.021). No differences were found in other white matter regions until 48 weeks. Histology indicated demyelination, axonal degeneration and coagulative necrosis in all injured white matter. DTI can serve as a promising tool for assessment of radiation induced white matter injury and regional radiosensitivity of white matter tracts.


Similar content being viewed by others
Abbreviations
- DTI:
-
Diffusion tensor MR imaging
- FA:
-
Fractional anisotropy
- ROI:
-
Region of interest
- RION:
-
Radiation induced optic neuropathy
- H&E:
-
Hematoxylin and eosin
- LFB:
-
Luxol fast blue
- NF:
-
Neural filament
References
Khong PL, Leung LH, Fung AS et al (2006) White matter anisotropy in post-treatment childhood cancer survivors: preliminary evidence of association with neurocognitive function. J Clin Oncol 24:884–890
Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE (2004) Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol 5:399–408
Bijl HP, van Luijk P, Coppes RP, Schippers JM, Konings AW, van Der Kogel AJ (2005) Regional differences in radiosensitivity across the rat cervical spinal cord. Int J Radiat Oncol Biol Phys 61:543–551
Qiu D, Kwong DL, Chan GC, Leung LH, Khong PL (2007) Diffusion tensor magnetic resonance imaging finding of discrepant fractional anisotropy between the frontal and parietal lobes after whole-brain irradiation in childhood medulloblastoma survivors: reflection of regional white matter radiosensitivity? Int J Radiat Oncol Biol Phys 69:846–851
Bodewitz ST, Keeren GJ (2011) Radiation-induced optic neuropathy. JBR-BTR 94:110–111
Miller NR (2004) Radiation-induced optic neuropathy: still no treatment. Clin Exp Ophthalmol 32:233–235
Stafford SL, Pollock BE, Leavitt JA et al (2003) A study on the radiation tolerance of the optic nerves and chiasm after stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 55:1177–1181
Lessell S (2004) Friendly fire: neurogenic visual loss from radiation therapy. J Neuroophthalmol 24:243–250
Basser PJ, Mattiello J, LeBihan D (1994) Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B 103:247–254
Mori S, Zhang J (2006) Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51:527–539
Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH (2003) Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 20:1714–1722
Wang S, Wu EX, Cai K, Lau HF, Cheung PT, Khong PL (2009) Mild hypoxic-ischemic injury in the neonatal rat brain: longitudinal evaluation of white matter using diffusion tensor MR imaging. AJNR Am J Neuroradiol 30:1907–1913
Wang S, Wu EX, Tam CN, Lau HF, Cheung PT, Khong PL (2008) Characterization of white matter injury in a hypoxic-ischemic neonatal rat model by diffusion tensor MRI. Stroke 39:2348–2353
Wang S, Wu EX, Qiu D, Leung LH, Lau HF, Khong PL (2009) Longitudinal diffusion tensor magnetic resonance imaging study of radiation-induced white matter damage in a rat model. Cancer Res 69:1190–1198
Wang S, Chen Y, Lal B, Ford E, Tryggestad E, Armour M, Yan K, Laterra J, Zhou J (2012) Evaluation of radiation necrosis and malignant glioma in rat models using diffusion tensor MR imaging. J Neurooncol 107:51–60
Chan KC, Khong PL, Cheung MM, Wang S, Cai KX, Wu EX (2009) MRI of late microstructural and metabolic alterations in radiation-induced brain injuries. J Magn Reson Imaging 29:1013–1020
Zhou JY, Tryggestad E, Wen ZB et al (2011) Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nat Med 17:130–134
Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S (2006) DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed 81:106–116
Gu J, Chan T, Zhang J, Leung AY, Kwong YL, Khong PL (2011) Whole-body diffusion-weighted imaging: the added value to whole-body MRI at initial diagnosis of lymphoma. AJR Am J Roentgenol 197:W384–W391
Gu J, Khong PL, Wang S, Chan Q, Law W, Zhang J (2011) Quantitative assessment of diffusion-weighted MR imaging in patients with primary rectal cancer: correlation with FDG-PET/CT. Mol Imaging Biol 13:1020–1028
Calvo W, Hopewell JW, Reinhold HS, Yeung TK (1988) Time- and dose-related changes in the white matter of the rat brain after single doses of X rays. Br J Radiol 61:1043–1052
Monje ML, Mizumatsu S, Fike JR, Palmer TD (2002) Irradiation induces neural precursor-cell dysfunction. Nat Med 8:955–962
Beaulieu C (2002) The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed 15:435–455
Wang S, Tryggestad E, Zhou T, Armour M, Wen Z, Fu DX, Ford E, van Zijl PC, Zhou J (2012) Assessment of MRI parameters as imaging biomarkers for radiation necrosis in the rat brain. Int J Radiat Oncol Biol Phys 83:e431–e436
Grossman R, Tyler B, Brem H, Eberhart CG, Wang S, Fu DX, Wen Z, Zhou J (2012) Growth properties of SF188/V+ human glioma in rats in vivo observed by magnetic resonance imaging. J Neurooncol 110(3):315–323
Wang S, Zhou J (2012) Diffusion tensor MR imaging of rat glioma models: a correlation study of MR imaging and histology. J Comput Assist Tomogr 36:739–744
Kim SK, Cho KO, Kim SY (2009) The plasticity of posterior communicating artery influences on the outcome of white matter injury induced by chronic cerebral hypoperfusion in rats. Neurol Res 31:245–250
Chan SO, Chow KL, Jen LS (1989) Postnatal development of the ipsilaterally projecting retinal ganglion cells in normal rats and rats with neonatal lesions. Brain Res Dev Brain Res 49:265–274
Perry VH, Henderson Z, Linden R (1983) Postnatal changes in retinal ganglion cell and optic axon populations in the pigmented rat. J Comp Neurol 219:356–368
Kerschensteiner M, Schwab ME, Lichtman JW, Misgeld T (2005) In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat Med 11:572–577
Richardson PM, Issa VM, Shemie S (1982) Regeneration and retrograde degeneration of axons in the rat optic nerve. J Neurocytol 11:949–966
Cowey A, Stoerig P, Williams C (1999) Variance in transneuronal retrograde ganglion cell degeneration in monkeys after removal of striate cortex: effects of size of the cortical lesion. Vision Res 39:3642–3652
Acknowledgments
The study is supported by University of Hong Kong Committee on Research and Conference grants (HKU7587/06M).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Silun Wang and Deqiang Qiu contributed equally to the study.
Rights and permissions
About this article
Cite this article
Wang, S., Qiu, D., So, KF. et al. Radiation induced brain injury: assessment of white matter tracts in a pre-clinical animal model using diffusion tensor MR imaging. J Neurooncol 112, 9–15 (2013). https://doi.org/10.1007/s11060-012-1031-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-012-1031-0