Abstract
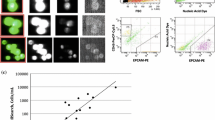
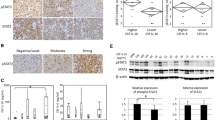
The role of cerebrospinal fluid (CSF) in the pathogenesis of meningiomas is unknown. Cell cultures from three human leptomeninges, five WHO grade I and seven grade II meningiomas were treated with remnant CSF from 22 patients with no central nervous system disease and normal cell indices. Cells were evaluated by CyQUANT for DNA synthesis/cell proliferation and by western blots for phosphorylation/activation of growth regulatory pathways activated in meningiomas including JAK1–STAT3, MEK1–p44/42MAPK, Akt–mTOR and Rb. Analysis of Caspase 3 activation and survivin was also performed. Finally, the effects of PDGF neutralizing antibody and cucurbitacin, a STAT3 inhibitor on CSF stimulation were tested. Compared to controls and the mitogen PDGF-BB, various CSF samples significantly stimulated DNA synthesis/cell proliferation in 20 and 22 week leptomeningeal cultures and all of the grade I and II meningioma cells tested. Collectively CSF samples, from multiple different patients, stimulated DNA synthesis in tests of 23 of 32 grade I and 18 of 28 grade II meningioma cells. CSF stimulated phosphorylation/activation of STAT3 and reduced p44/42 MAPK in the leptomeningeal, all three grade I and 1 of three grade II meningioma cells. CSF did not affect Caspase 3 activity or survivin levels. PDGF neutralizing antibody had no effect on CSF stimulation but cucurbitacin blocked PDGF and CSF stimulation. While there are limitations to the CSF available since they were not from “normal” volunteers, the studies suggest that, in some settings, CSF is potentially mitogenic to leptomeningeal and meningioma cells and may act, in part, via activation of STAT3.






Similar content being viewed by others
References
Gato A, Desmond ME (2009) Why the embryo still matters: CSF and neuroepithelium as interdependent regulators of embryonic brain growth, morphogenesis and histogenesis. Dev Biol 327:263–272
Johanson CE, Duncan JA, Klinge PM, Brinker T, Stopa EG, Silverberg GD (2008) Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res 5:10–42
Johnson MD, Woodard A, Okediji EJ, Toms SA, Allen GS (2002) Lovastatin is a potent inhibitor of meningioma cell proliferation: evidence for inhibition of a mitogen associated protein kinase. J Neurooncol 56:133–142
Johnson MD, Okediji E, Woodard A (2004) Transforming growth factor-β effects on meningioma cell proliferation and signal transduction pathways. J Neurooncol 66:9–166
Johnson MD, Woodard A, Kim P, Frexes-Steed M (2001) Evidence for mitogen associated protein kinase activation and transduction of mitogenic signals from platelet derived growth factor in human meningioma cells. J Neurosurg 94:303–310
Johnson MD, Okediji E, Woodard A, Toms SA (2002) Evidence for phosphatidylinositol 3-kinase Akt- p70S6K pathway activation and transduction of mitogenic signals by platelet derived growth factor in human meningioma cells. J Neurosurg 97:668–675
Magrassi L, De-Fraja C, Conti L, Butti G, Infuso L, Govoni S et al (1999) Expression of the JAK and STAT superfamilies in human meningiomas. J Neurosurg 91:440–446
Johnson MD, Fran Vito F, O’Connell MJ, Pilcher W (2009) Bone morphogenetic protein-4 and receptors are expressed in the leptomeninges and meningiomas and signal via MAPK. J Neuropathol Exp Neurol 68:1173–1184
Buccoliero AM, Castiglione F, Degl’Innocenti DR, Gheri CF, Garbini F, Taddei A et al (2007) NF2 gene expression in sporadic meningiomas: relation to grades or histotypes real time-PCR study. Neuropathology 27:36–42
Gusella JF, Ramesh V, MacCollin M et al (1999) Merlin: the neurofibromatosis 2 tumor suppressor. Biochem Biophys Acta 1423:M29–M36
Johnson MD, O’Connell M, Vito F, Bakos RS (2009) Increased STAT-3 and synchronous activation of Raf-1-MEK-1-MAPK, and Phosphatidylinositol 3-Kinase-Akt-mTOR Pathways in Atypical and Anaplastic Meningiomas. J Neurooncol 92:129–135
Zhang MX, Zhao X, Wang ZG, Zhao WM, Wang YS (2010) Constitutive activation of signal transducer and activator of transcription 3 regulates expression of vascular endothelial growth factor in human meningioma differentiation. J Cancer Res Clin Oncol 136:981–988
Blaskovich MA et al (2003) Discovery of JSI-124 (curcurbitacin 1), a selective Janus kinase/signal transducer and transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res 63:1270–1279
Germain D, Frank DA (2007) Targeting the cytoplasmic and nuclear functions of signal transducers and activators of transcription 3 for cancer therapy. Clin Cancer Res 13:5665–5669
Klampfer L (2006) Signal transducers and activators of transcription (STATs): novel targets of chemopreventive and chemotherapeutic drugs. Curr Cancer Drugs Targets 6:107–121
Kortylewski M, Hua Y (2007) STAT3 as a potential target for cancer immunotherapy. J Immunother 30:131–139
Silva CM (2004) Role of STATs as downstream signal transducers in Src family kinase-mediated tumorigenesis. Oncogene 23:8017–8023
Schrell UMH, Koch HU, Marschlaek R, Schrauzer T et al (1998) Formation of autocrine loops in human cerebral meningioma tissue by leukemia inhibitor factor, interleukin-6, and oncostatin M: inhibition of meningioma cell growth in vitro by recombinant oncostatin M. J Neurosurg 88:541–548
Todo T, Adams EF, Rafferty B, Fahlbusch R, Dingermann T et al (1994) Secretion of interleukin-6 by meningioma cells: possible autocrine inhibitory regulation of neoplastic cell growth. J Neurosurg 81:394–401
Mawrin C, Sasse T, Kirches E et al (2005) Different activation of mitogen activated protein kinase and Akt signaling is associated with aggressive phenotype of human meningiomas. Clin Cancer Res 11:4074–4082
Kellie S, Craggs G, Bird IN, Jones GE (2003) The tyrosine phosphatase DEP-1 induces cytoskeletal rearrangements, abberant cell-substratum interactions and a reduction in cell proliferation. J Cell Sci 117:618
Chen L, Sung S–S, Yip MLR, Lawrence HR, Ren Y, Guida WC, Sebti SM, Lawrence NJ, Wu J (2006) Discovery of a novel Shp2 protein tyrosine phosphatase inhibitor. Mol Pharmacol 70:562–570
Navis AC, Achepens JTG, van Huijsduijenen RH, Wesseling P, Hendriks WJAJ (2010) Protein tyrosine phosphatases in glioma biology. Acta Neuropathol 119:157–175
Dattatreyamurty B, Roux E, Horbinski C, Kaplan PL, Robak LA, Beck HN et al (2001) Cerebrospinal fluid contains biologically active bone morphogenic protein-7. Exp Neurol 172:273–281
Douglas MR, Daniel M, Lagord C, Akinwunmi J, Jackowski A, Cooper C et al (2007) High CSF transforming growth factor beta levels after subarachnoid haemorrhage; association with chronic communicating hydrocephalus. J Neurol Neurosurg Psychiatry 80:545–550
Johnson MD, Gold LI, Moses HL (1992) Evidence for TGF-β expression in human leptomeningeal cells and TGF-β-like activity in human cerebrospinal fluid. Lab Invest 67:360–368
Johnson MD, Kim P, Tourtelotte W, Federspiel CF (2004) Transforming growth factor B and monocyte chemotactic protein-1 are elevated in cerebrospinal fluid of immunocompromised patients with HIV-1 infection. J NeuroAIDS 2:33–43
Li X, Miyajima M, Jiang C, Arai H (2007) Expression of TGF-Bs and TGF-B type II receptor in cerebrospinal fluid of patients with idiopathic normal pressure hydrocephalus. Neurosci Lett 413:141–144
Rota E, Bellone G, Rocca P, Bergamasco B, Emanuelli G, Ferrero P (2006) Increased intrathecal TGF-B1 but not IL-12, IFN-and IL-10 levels in Alzheimer’s disease patients. Neurol Sci 27:33–39
Nister M, Enbland P, Backstrom G, Soderman T, Persson L, Heldin CH et al (1994) Platelet-derived growth factor (PDGF) in neoplastic and non-neoplastic cystic lesions of the central nervous system and in the cerebrospinal fluid. Br J Cancer 69:952–956
Sagoh M, Yoshida K, Wakamoto H, Kamiguchi H, Otani M, Shiobara R et al (1995) Accumulation of nerve growth factor in cerebrospinal fluid and biological activity following neurosurgery. Neurol Med Chir 35:431–437
Hannenken A, Frautschy S, Galasko D, Baird A (1995) A fibroblast growth factor binding protein in human cerebrospinal fluid. Neuroreport 6:886–888
Sarchielli P, Di Filippo M, Ercloani MV et al (2008) Fibroblast growth factor-2 levels are elevated in the cerebrospinal fluid of multiple sclerosis patients. Neurosci Lett 435:223–228
Van Setten GB, Edstrom L, Stibler H, Rasmussen S, Schultz G (1999) Levels of transforming growth factor alpha (TGF-α) in human cerebrospinal fluid. Int J Dev Neurosci 17:131–134
Johnson M, Toms S (2006) Mitogenic signal transduction pathways in meningiomas: novel targets for meningioma chemotherapy? J Neuropathol Exp Neurol 64:1029–1036
Ragel BT, Jensen RL (2010) Aberrant signaling pathways in meningiomas. J Neurooncol 99:315–324
Jaaskelainen J (1986) Seemingly complete removal of histologically benign intracranial meningioma: late recurrence rate and factors predicting recurrence in 637 patients. A multivariate analysis. Surg Neurol 26:461–469
Stafford SL, Perry A, Suman VJ et al (1998) Primarily resected meningiomas: outcomes and prognostic factors in 581 Mayo Clinic patients, 1978 through 1988. Mayo Clin Proc 73:936–942
Perry A, Stafford SL, Scheithauer BW et al (1997) Meningioma grading: an analysis of histologic parameters. Am J Surg Pathol 21:1455–1465
Pham MH, Zada G, Mosich GM, Chen TC, Giannotta SL, Wang K, Mack WJ (2011) Molecular genetics of meningiomas: a systematic review of the current literature and potential basis for future treatment paradigms. Neurosurg Focus 30:E7
Conflict of interest
The authors declare they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Johnson, M.D., O’Connell, M., Facik, M. et al. Cerebrospinal fluid stimulates leptomeningeal and meningioma cell proliferation and activation of STAT3. J Neurooncol 107, 121–131 (2012). https://doi.org/10.1007/s11060-011-0736-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-011-0736-9