Abstract
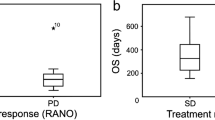
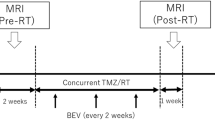
The purpose of this study was to assess the usefulness of diffusion weighted imaging as an additional imaging biomarker for treatment response in recurrent/progressive malignant gliomas treated with bevacizumab alone or in combination with other chemotherapeutic agents. Twenty patients treated with bevacizumab alone or concurrent chemotherapy were followed up with serial MR imaging. Volume and ADC values of contrast enhancing lesion (CELvol, CELADC) and also of non-enhancing lesion (NELvol, NELADC) were obtained. CELvol showed a progressive decrease in non-progressors with a median percentage change of −73.2% (P = 0.001) as compared to −33.4% for progressors by 1 year/last imaging (P = 0.382). NELvol also showed a decrease in non-progressors on follow up imaging though only significant for 3 months follow up (P = 0.042). In progressors, CELvol and NELvol showed initial decrease followed by slight increase by 1 year/last imaging though not significant (P value of 0.382 and 0.46, respectively). CELADC and NELADC in non-progressors did not show any statistically significant change though there was slight trend for positive percent change especially for CELADC by 1 year/last imaging follow up study (P value of 0.077 and 0.339, respectively). Progressors showed a progressive negative percent change of CELADC and NELADC. In progressors, NELADC decreased at 6 weeks (P = 0.054), 3 months (P = 0.023) and 1 year/last (P = 0.078) as compared to baseline study and was also statistically significant as compared to non-progressors at 6 weeks (P = 0.047) and 3 months (P = 0.025). CELADC and NELADC appear to follow different trends over time for non-progressors and progressors with a stable to slightly progressive increase in non-progressors and a progressive decrease in progressors, especially early on. These findings suggest that DWI may be used as an additional imaging biomarker for early treatment response.








Similar content being viewed by others
References
Miller AB, Hoogstraten B, Staquet M et al (1981) Reporting results of cancer treatment. Cancer 47:207–214
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Macdonald DR, Cascino TL, Schold SC et al (1990) Response criteria for phase II studies of malignant glioma. J Clin Oncol 8:1277–1280
Henson JW, Ulmer S, Harris GJ (2008) Brain tumor imaging in clinical trials. AJNR Am J Neuroradiol 29(3):419–424
Sorensen AG, Batchelor TT, Wen PY et al (2008) Response criteria for glioma. Nat Clin Pract Oncol 5:634–644
Le Bihan D, Breton E, Lallemand D et al (1986) MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 161:401–407
Tien RD, Felsberg GJ, Friedman H et al (1994) MR imaging of high-grade cerebral gliomas: value of diffusion weighted echoplanar pulse sequences. AJR Am J Roentgenol 162:671–677
Brunberg JA, Chenevert TL, McKeever PE et al (1995) In vivo MR determination of water diffusion coefficients and diffusion anisotropy: correlation with structural alteration in gliomas of the cerebral hemispheres. AJNR Am J Neuroradiol 16:361–371
Noguchi K, Watanabe N, Nagayoshi T et al (1999) Role of diffusion weighted echo-planar MRI in distinguishing between brain abscess and tumor: a preliminary report. Neuroradiology 41:171–174
Gupta RK, Sinha U, Cloughesy TF et al (1999) Inverse correlation between choline magnetic resonance spectroscopy signal intensity and the apparent diffusion coefficient in human glioma. Magn Reson Med 41:2–7
Stark Vance V (2005) Bevacizumab (Avastin) and CPT-11 (Camptosar) in the treatment of relapsed malignant glioma. Neurooncology 7:369
Pope WB, Lai A, Nghiemphu P et al (2006) MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology 66:1258–1260
Vredenburgh JJ, Desjardins A, Herndon JE et al (2007) Bevacizumab, a monoclonal antibody to vascular endothelial growth factor (VEGF), and irinotecan for treatment of malignant glioma. Clin Cancer Res 13:1253–1259
Holash J, Maisonpierre PC, Compton D et al (1999) Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 284:1994–1998
Kunkel P, Ulbricht U, Bohlen O et al (2001) Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res 61:6624–6628
Rubenstein JL, Kim J, Ozawa T et al (2000) Anti-VEGF antibody treatment for glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia 2:306–314
Norden AD, Young GS, Setayesh K (2008) Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology 70(10):779–787
Fischer I, Cunliffe CH, Bollo RJ (2008) High-grade glioma before and after treatment with radiation and Avastin: initial observations. Neurooncology 10(5):700–708
Batchelor TT, Sorensen AG et al (2007) AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11:83–95
Moffat BA, Chenevert TL, Meyer CR et al (2006) The functional diffusion map: an imaging biomarker for the early prediction of cancer treatment outcome. Neoplasia 8:259–267
Ellika S, Jain R, Scarpace L, et al. (2007) Role of functional diffusion maps in predicting progression of low grade gliomas. In: Proceedings of American society of neuroradiology annual meeting 2007, p 242
Ananthnarayan S, Bahng J, Roring J et al (2008) Time course of imaging changes of GBM during extended bevacizumab treatment. J Neurooncol 88:339–347
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jain, R., Scarpace, L.M., Ellika, S. et al. Imaging response criteria for recurrent gliomas treated with bevacizumab: Role of diffusion weighted imaging as an imaging biomarker. J Neurooncol 96, 423–431 (2010). https://doi.org/10.1007/s11060-009-9981-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-009-9981-6