Abstract
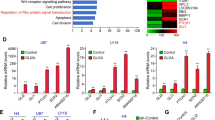
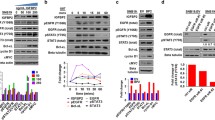
HMGB1 (high mobility group box 1 protein) is a nuclear protein that can also act as an extracellular trigger of inflammation, proliferation and migration, mainly through RAGE (the receptor for advanced glycation end products); HMGB1–RAGE interactions have been found to be important in a number of cancers. We investigated whether HMGB1 is an autocrine factor in human glioma cells. Western blots showed HMGB1 and RAGE expression in human malignant glioma cell lines. HMGB1 induced a dose-dependent increase in cell proliferation, which was found to be RAGE-mediated and involved the MAPK/ERK pathway. Moreover, in a wounding model, it induced a significant increase in cell migration, and RAGE-dependent activation of Rac1 was crucial in giving the tumour cells a motile phenotype. The fact that blocking DNA replication with anti-mitotic agents did not reduce the distance migrated suggests the independence of the proliferative and migratory effects. We also found that glioma cells contain HMGB1 predominantly in the nucleus, and cannot secrete it constitutively or upon stimulation; however, necrotic glioma cells can release HMGB1 after it has translocated from the nucleus to cytosol. These findings provide the first evidence supporting the existence of HMGB1/RAGE signalling pathways in human glioblastoma cells, and suggest that HMGB1 may play an important role in the relationship between necrosis and malignancy in glioma tumours by acting as an autocrine factor that is capable of promoting the growth and migration of tumour cells.







Similar content being viewed by others
References
Lotze MT, Tracey KJ (2005) High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 5:331–342
Müller S, Ronfani L, Bianchi ME (2004) Regulated expression and subcellular localization of HMGB1, a chromatin protein with a cytokine function. J Intern Med 255:332–343
Yang H, Wang H, Czura CJ, Tracey KJ (2005) The cytokine activity of HMGB1. J Leukoc Biol 78:1–8
Ulloa L, Messmer D (2006) High-mobility group box 1 (HMGB1) protein: friend and foe. Cytokine Growth Factor Rev 17:189–201
Bianchi ME (2004) Significant (re)location: how to use chromatin and/or abundant proteins as messages of life and death. Trends Cell Biol 14:287–293
Erlandsson Harris H, Andersson U (2004) The nuclear protein HMGB1 as a proinflammatory mediator. Eur J Immunol 34:1503–1512
Wang H, Yang H, Tracey KJ (2004) Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med 255:320–331
Raucci A, Palumbo R, Bianchi ME (2007) HMGB1: a signal of necrosis. Autoimmunity 40:285–289
Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, Stern DM, Nawroth PP (2005) Understanding RAGE, the receptor for advanced glycation end products. J Mol Med 83:876–886
Huttunen HJ, Rauvala H (2004) Amphoterin as an extracellular regulator of cell motility: from discovery to disease. J Intern Med 255:351–366
Ellerman JE, Brown CK, de Vera M, Zeh HJ, Billiar T, Rubartelli A, Lotze MT (2007) Masquerader: high mobility group box-1 and cancer. Clin Cancer Res 13:2836–2848
Kuniyasu H, Chihara Y, Takahashi T (2003) Co-expression of receptor for advanced glycation end products and the ligand amphoterin associates closely with metastasis of colorectal cancer. Oncol Rep 10:445–448
Huttunen HJ, Fages C, Kuja-Panula J, Ridley AJ, Rauvala H (2002) Receptor for advanced glycation end products-binding COOH-terminal motif of amphoterin inhibits invasive migration and metastasis. Cancer Res 62:4805–4811
Bartling B, Hofmann HS, Weigle B, Silber RE, Simm A (2005) Down-regulation of the receptor for advanced glycation end-products (RAGE) supports non-small cell lung carcinoma. Carcinogenesis 26:293–301
Riuzzi F, Sorci G, Donato R (2006) The amphoterin (HMGB1)/receptor for advanced glycation end products (RAGE) pair modulates myoblast proliferation, apoptosis, adhesiveness, migration, and invasiveness. Functional inactivation of RAGE in L6 myoblasts results in tumor formation in vivo. J Biol Chem 281:8242–8253
Lotze MT, DeMarco RA (2003) Dealing with death: HMGB1 as a novel target for cancer therapy. Curr Opin Investig Drugs 4:1405–1409
Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavenee WK (2002) The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol 61:215–225
Tysnes BB, Mahesparan R (2001) Biological mechanisms of glioma invasion and potential therapeutic targets. J Neurooncol 53:129–147
Hulleman E, Helin K (2005) Molecular mechanisms in gliomagenesis. Adv Cancer Res 94:1–27
Sparatore B, Passalacqua M, Patrone M, Melloni E, Pontremoli S (1996) Extracellular high-mobility group 1 protein is essential for erythroleukaemia cell differentiation. Biochem J 320:253–256
Sparatore B, Patrone M, Passalacqua M, Pedrazzi M, Ledda S, Pontremoli S, Melloni E (2005) Activation of A431 human carcinoma cell motility by extracellular high-mobility group box 1 protein and epidermal growth factor stimuli. Biochem J 389:215–221
Riboni L, Viani P, Bassi R, Giussani P, Tettamanti G (2001) Basic fibroblast growth factor-induced proliferation of primary astrocytes. J Biol Chem 276:12797–12804
Bassi R, Anelli V, Giussani P, Tettamanti G, Viani P, Riboni L (2006) Sphingosine-1-phosphate is released by cerebellar astrocytes in response to bFGF and induces astrocyte proliferation through Gi-protein-coupled receptors. Glia 53:621–630
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Rubinfeld H, Seger R (2005) The ERK cascade: a prototype of MAPK signaling. Mol Biotechnol 31:151–174
Wallace EM, Lyssikatos JP, Yeh T, Winkler JD, Koch K (2005) Progress towards therapeutic small molecule MEK inhibitors for use in cancer therapy. Curr Top Med Chem 5:215–229
Taguchi A, Blood DC, Del Toro G et al (2000) Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature 405:354–360
Salhia B, Tran NL, Symons M, Winkles JA, Rutka JT, Berens ME (2006) Molecular pathways triggering glioma cell invasion. Expert Rev Mol Diagn 6:613–626
Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418:191–195
Bianchi ME, Manfredi A (2004) Chromatin and cell death. Biochim Biophys Acta 1677:181–186
Mimeault M (2002) New advances on structural and biological functions of ceramide in apoptotic/necrotic cell death and cancer. FEBS Lett 530:9–16
Ogretmen B, Hannun YA (2004) Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer 4:604–616
Mochizuki T, Asai A, Saito N, Tanaka S, Katagiri H, Asano T, Nakane M, Tamura A, Kuchino Y, Kitanaka C, Kirino T (2002) Akt protein kinase inhibits non-apoptotic programmed cell death induced by ceramide. J Biol Chem 277:2790–2797
Meloche S, Pouysségur J (2007) The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene 26:3227–3239
Sanson M, Thillet J, Hoang-Xuan K (2004) Molecular changes in gliomas. Curr Opin Oncol 16:607–613
Ishihara K, Tsutsumi K, Kawane S, Nakajima M, Kasaoka T (2003) The receptor for advanced glycation end-products (RAGE) directly binds to ERK by a D-domain-like docking site. FEBS Lett 550:107–113
Giese A, Loo MA, Tran N, Haskett D, Coons SW, Berens ME (1996) Dichotomy of astrocytoma migration and proliferation. Int J Cancer 67:275–282
Fages C, Nolo R, Huttunen HJ, Eskelinen E, Rauvala H (2000) Regulation of cell migration by amphoterin. J Cell Sci 113:611–620
Qiu RG, Chen J, Kirn D, McCormick F, Symons M (1995) An essential role for Rac in Ras transformation. Nature 374:457–459
Chan AY, Coniglio SJ, Chuang YY, Michaelson D, Knaus UG, Philips MR, Symons M (2005) Roles of the Rac1 and Rac3 GTPases in human tumor cell invasion. Oncogene 24:7821–7829
Chuang YY, Tran NL, Rusk N, Nakada M, Berens ME, Symons M (2004) Role of synaptojanin 2 in glioma cell migration and invasion. Cancer Res 64:8271–8275
Salhia B, Rutten F, Nakada M, Beaudry C, Berens M, Kwan A, Rutka JT (2005) Inhibition of Rho-kinase affects astrocytoma morphology, motility, and invasion through activation of Rac1. Cancer Res 65:8792–8800
Passalacqua M, Zicca A, Sparatore B, Patrone M, Melloni E, Pontremoli S (1997) Secretion and binding of HMG1 protein to the external surface of the membrane are required for murine erythroleukemia cell differentiation. FEBS Lett 400:275–279
Kuniyasu H, Yano S, Sasaki T, Sasahira T, Sone S, Ohmori H (2005) Colon cancer cell-derived high mobility group 1/amphoterin induces growth inhibition and apoptosis in macrophages. Am J Pathol 166:751–760
Wähämaa H, Vallerskog T, Qin S, Lunderius C, LaRosa G, Andersson U, Harris HE (2007) HMGB1-secreting capacity of multiple cell lineages revealed by a novel HMGB1 ELISPOT assay. J Leukoc Biol 81:129–136
Brezniceanu ML, Volp K, Bosser S, Solbach C, Lichter P, Joos S, Zornig M (2003) HMGB1 inhibits cell death in yeast and mammalian cells and is abundantly expressed in human breast carcinoma. FASEB J 17:1295–1297
Ditsworth D, Zong WX, Thompson CB (2007) Activation of poly(ADP)-ribose polymerase (PARP-1) induces release of the pro-inflammatory mediator HMGB1 from the nucleus. J Biol Chem 282:17845–17854
Wharton B, McNelis U, Bell HS, Whittle IR (2000) Expression of poly(ADP-ribose) polymerase and distribution of poly(ADP-ribosyl)ation in glioblastoma and in a glioma multicellular tumour spheroid model. Neuropathol Appl Neurobiol 26:528–535
Raza SM, Lang FF, Aggarwal BB, Fuller GN, Wildrick DM, Sawaya R (2002) Necrosis and glioblastoma: a friend or a foe? A review and a hypothesis. Neurosurgery 51:2–12
Acknowledgements
This work was supported in part by grants from the Italian Ministry of University and Scientific and Technological Research PRIN and FIRST to L.R.
Author information
Authors and Affiliations
Corresponding author
Additional information
Rosaria Bassi and Paola Giussani contributed equally to this study.
Rights and permissions
About this article
Cite this article
Bassi, R., Giussani, P., Anelli, V. et al. HMGB1 as an autocrine stimulus in human T98G glioblastoma cells: role in cell growth and migration. J Neurooncol 87, 23–33 (2008). https://doi.org/10.1007/s11060-007-9488-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-007-9488-y