Abstract
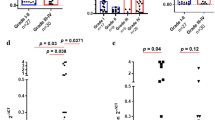
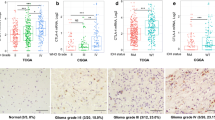
In this study, we investigated the mRNA and protein expression of nine tumour antigens in human glioblastoma multiforme with a view to their possible use in dendritic cell-based immunotherapy. Expression of ALK, EGFRvIII, GALT3, gp100, IL-13Rα2, MAGE-A3, NA17-A, TRP-2 and tyrosinase were studied by real-time RT-PCR on frozen tissues using a series of 47 tumour samples from patients with glioblastoma. Results were compared with non-neoplastic brain expression or glioblastoma samples with very low levels of expression near the limits of detection for EGFRvIII and MAGE-A3, as these latter two antigens were not detected in non-neoplastic brain. Tumour antigens showing a 5-fold increase in mRNA expression were considered as positive, and only antigens displaying an mRNA over-expression in a significant number of cases were analysed by immunohistochemistry on paraffin-embedded sections. Using real time RT-PCR, we found EGFRvIII, gp100, IL-13Rα2 and TRP-2 to be positive in 64, 38, 32 and 21% of cases, respectively. While we observed no over-expression for ALK, GALT3 and tyrosinase, 3 samples out of 47 were positive for MAGE-3 and 1 sample for NA17-A. More than 25% of tumour cells showed strong protein expression in 13, 34, 85 and 96% of GBM samples for gp100, TRP-2, EGFRvIII and IL-13Rα2, respectively. Interestingly, protein expression of at least 3 antigens was observed in 38% of cases. These results point out the importance of EGFRvIII, IL-13Rα2 and, to a less extent gp100 and TRP-2, for developing an immunotherapy strategy against glioblastoma.
Similar content being viewed by others
Abbreviations
- CTL:
-
Cytotoxic T cell
- GBM:
-
Glioblastoma multiforme
- MHC:
-
Major histocompatibility complex
References
Holland EC (2000) Glioblastoma multiforme: the terminator. Proc Natl Acad Sci USA 97:6242–6244
Kikuchi T, Akasaki Y, Irie M et al (2001) Results of a phase I clinical trial of vaccination of glioma patients with fusions of dendritic and glioma cells. Cancer Immunol Immunother 50:337–344
Yu JS, Wheeler CJ, Zeltzer PM et al (2001) Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res 61:842–847
Yamanaka R, Abe T, Yajima N et al (2003) Vaccination of recurrent glioma patients with tumour lysate-pulsed dendritic cells elicits immune responses: results of a clinical phase I/II trial. Br J Cancer 89:1172–1179
Kikuchi T, Akasaki Y, Abe T et al (2004) Vaccination of glioma patients with fusions of dendritic and glioma cells and recombinant human interleukin 12. J Immunother 27:452–459
Rutkowski S, De Vleeschouwer S, Kaempgen E et al (2004) Surgery and adjuvant dendritic cell-based tumour vaccination for patients with relapsed malignant glioma, a feasibility study. Br J Cancer 91:1656–1662
Yu JS, Liu G, Ying H et al (2004) Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res 64:4973–4979
Liau LM, Prins RM, Kiertscher SM et al (2005) Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res 11:5515–5525
Yamanaka R, Homma J, Yajima N et al (2005) Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res 11:4160–4167
Chi DD, Merchant RE, Rand R et al (1997) Molecular detection of tumor-associated antigens shared by human cutaneous melanomas and gliomas. Am J Pathol 150:2143–2152
Joshi BH, Plautz GE, Puri RK (2000) Interleukin-13 receptor alpha chain: a novel tumor-associated transmembrane protein in primary explants of human malignant gliomas. Cancer Res 60:1168–1172
Bernard J, Treton D, Vermot-Desroches C et al (2001) Expression of interleukin 13 receptor in glioma and renal cell carcinoma: IL13Ralpha2 as a decoy receptor for IL13. Lab Invest 81:1223–1231
Liu G, Khong HT, Wheeler CJ et al (2003) Molecular and functional analysis of tyrosinase-related protein (TRP)-2 as a cytotoxic T lymphocyte target in patients with malignant glioma. J Immunother 26:301–312
Kawakami M, Kawakami K, Takahashi S et al (2004) Analysis of interleukin-13 receptor alpha2 expression in human pediatric brain tumors. Cancer 101:1036–1042
Liu G, Ying H, Zeng G et al (2004) HER-2, gp100, and MAGE-1 are expressed in human glioblastoma and recognized by cytotoxic T cells. Cancer Res 64:4980–4986
Hoftberger R, Aboul-Enein F, Brueck W et al (2004) Expression of major histocompatibility complex class I molecules on the different cell types in multiple sclerosis lesions. Brain Pathol 14:43–50
Iwahara T, Fujimoto J, Wen D et al (1997) Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene 14:439–449
Tsuda N, Nonaka Y, Shichijo S et al (2002) UDP-Gal: betaGlcNAc beta1, 3-galactosyltransferase, polypeptide 3 (GALT3) is a tumour antigen recognised by HLA-A2-restricted cytotoxic T lymphocytes from patients with brain tumour. Br J Cancer 87:1006–1012
Dirks WG, Fahnrich S, Lis Y et al (2002) Expression and functional analysis of the anaplastic lymphoma kinase (ALK) gene in tumor cell lines. Int J Cancer 100:49–56
Powers C, Aigner A, Stoica GE et al (2002) Pleiotrophin signaling through anaplastic lymphoma kinase is rate-limiting for glioblastoma growth. J Biol Chem 277:14153–14158
Guilloux Y, Lucas S, Brichard VG et al (1996) A peptide recognized by human cytolytic T lymphocytes on HLA-A2 melanomas is encoded by an intron sequence of the N-acetylglucosaminyltransferase V gene. J Exp Med 183:1173–1183
Sahin U, Koslowski M, Tureci O et al (2000) Expression of cancer testis genes in human brain tumors. Clin Cancer Res 6:3916–3922
Scarcella DL, Chow CW, Gonzales MF et al (1999) Expression of MAGE and GAGE in high-grade brain tumors: a potential target for specific immunotherapy and diagnostic markers. Clin Cancer Res 5:335–341
Wikstrand CJ, Hale LP, Batra SK et al (1995) Monoclonal antibodies against EGFRvIII are tumor specific and react with breast and lung carcinomas and malignant gliomas. Cancer Res 55:3140–3148
Feldkamp MM, Lala P, Lau N et al (1999) Expression of activated epidermal growth factor receptors, Ras-guanosine triphosphate, and mitogen-activated protein kinase in human glioblastoma multiforme specimens. Neurosurgery 45:1442–1453
Shinojima N, Tada K, Shiraishi S et al (2003) Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res 63:6962–6970
Aldape KD, Ballman K, Furth A et al (2004) Immunohistochemical detection of EGFRvIII in high malignancy grade astrocytomas and evaluation of prognostic significance. J Neuropathol Exp Neurol 63:700–707
Biernat W, Huang H, Yokoo H et al (2004) Predominant expression of mutant EGFR (EGFRvIII) is rare in primary glioblastomas. Brain Pathol 14:131–136
Nishikawa R, Sugiyama T, Narita Y et al (2004) Immunohistochemical analysis of the mutant epidermal growth factor, deltaEGFR, in glioblastoma. Brain Tumor Pathol 21:53–56
Steiner HH, Bonsanto MM, Beckhove P et al (2004) Antitumor vaccination of patients with glioblastoma multiforme: a pilot study to assess feasibility, safety, and clinical benefit. J Clin Oncol 22:4272–4281
Arjona D, Bello MJ, Alonso ME et al (2005) Molecular analysis of the EGFR gene in astrocytic gliomas: mRNA expression, quantitative-PCR analysis of non-homogeneous gene amplification and DNA sequence alterations. Neuropathol Appl Neurobiol 31:384–394
Heimberger AB, Hlatky R, Suki D et al (2005) Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res 11:1462–1466
Debinski W, Gibo DM, Slagle B et al (1999) Receptor for interleukin 13 is abundantly and specifically over-expressed in patients with glioblastoma multiforme. Int J Oncol 15:481–486
Ekstrand AJ, Longo N, Hamid ML et al (1994) Functional characterization of an EGF receptor with a truncated extracellular domain expressed in glioblastomas with EGFR gene amplification. Oncogene 9:2313–2320
Moscatello DK, Holgado-Madruga M, Godwin AK et al (1995) Frequent expression of a mutant epidermal growth factor receptor in multiple human tumors. Cancer Res 55:5536–5539
Hershey GK (2003) IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol 111:677–690; quiz 691
Sanai N, Alvarez-Buylla A, Berger MS (2005) Neural stem cells and the origin of gliomas. N Engl J Med 353:811–822
Liu G, Akasaki Y, Khong HT et al (2005) Cytotoxic T cell targeting of TRP-2 sensitizes human malignant glioma to chemotherapy. Oncogene 24(33):5226–5234
Wu AH, Xiao J, Anker L et al (2006) Identification of EGFRvIII-derived CTL epitopes restricted by HLA A0201 for dendritic cell based immunotherapy of gliomas. J Neurooncol 76:23–30
Okano F, Storkus WJ, Chambers WH et al (2002) Identification of a novel HLA-A*0201-restricted, cytotoxic T lymphocyte epitope in a human glioma-associated antigen, interleukin 13 receptor alpha2 chain. Clin Cancer Res 8:2851–2855
Lorimer IA (2002) Mutant epidermal growth factor receptors as targets for cancer therapy. Curr Cancer Drug Targets 2:91–102
Fay JW, Palucka AK, Paczesny S et al (2006) Long-term outcomes in patients with metastatic melanoma vaccinated with melanoma peptide-pulsed CD34(+) progenitor-derived dendritic cells. Cancer Immunol Immunother 55(10):1209–1218
Acknowledgements
We thank the Neurosurgery Department (Hôpital Pontchaillou, Rennes) for providing us with tumour samples, as well as S. Moiteaux, P. Bellaud, F. Jouan, and A.␣Denais for their technical assistance. M.S.N. Carpenter post-edited the English style. This study was supported by Grants Nos. PHRC 2003, CPER 2000-2006 Région Bretagne.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saikali, S., Avril, T., Collet, B. et al. Expression of nine tumour antigens in a series of human glioblastoma multiforme: interest of EGFRvIII, IL-13Rα2, gp100 and TRP-2 for immunotherapy. J Neurooncol 81, 139–148 (2007). https://doi.org/10.1007/s11060-006-9220-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-006-9220-3