Summary
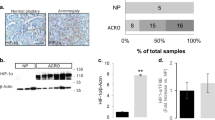
Hypoxia appears to be causatively related to pituitary adenoma. Currently, no biomarkers are available for the postoperative assessment of hypoxia in patient samples. Since the cAMP response element binding protein (CREB) is phosphorylated under hypoxic conditions, we examined whether CREB phosphorylation levels may be exploited as a novel biomarker for hypoxia in pituitary adenoma tissues. HP-75 human pituitary adenoma cells were incubated in 21% or 1% oxygen (normoxia and hypoxia, respectively), and Western blotting was employed to compare the levels of CREB and phosphorylated CREB (p-CREB). Our results show that p-CREB levels are significantly elevated under 1% oxygen, whereas the total CREB concentration remains unchanged. We further tested whether this phosphorylation is applicable as a marker of hypoxia in pituitary adenoma tissues removed by transsphenoidal surgery from 45 patients (32 females and 13 males, 22–78 years old). Fluorescence double immunohistochemistry data revealed that p-CREB in adenoma tissues is significantly elevated, and displays a positive correlation with Knosp grading (Spearman rank correlation; P = 0.0483, r = 0.3412), but no significant association with tumor subtype (Kruskal–Wallis analysis, CREB, P = 0.1072; p-CREB, P = 0.1888; phosphorylation ratio, P = 0.4916). Our findings collectively suggest that CREB phosphorylation may be employed as an in situ marker for hypoxia.␣Moreover, hypoxia and/or phosphorylation of CREB are associated with the cell invasiveness of pituitary adenomas.
Similar content being viewed by others
References
Vidal S, Horvath E, Kovacs K, Kuroki T, Lloyd RV, Scheithauer BW, Expression of hypoxia-inducible factor-1alpha (HIF-1alpha) in pituitary tumours Histol Histopathol 18: 679–686, 2003
Gronroos T, Bentzen L, Marjamaki P, Murata R, Horsman M R, Keiding S, Eskola O, Haaparanta M, Minn H, Solin O, Comparison of the biodistribution of two hypoxia markers [18F]FETNIM and [18F]FMISO in an experimental mammary carcinoma Eur J Nucl Med Mol Imaging 31: 513–520, 2004
Hofer SO, Mitchell GM, Penington AJ, Morrison WA, Romeomeeuw R, Keramidaris E, Palmer J, Knight KR: The use of pimonidazole to characterise hypoxia in the internal environment of an in vivo tissue engineering chamber. Br J Plast Surg 58: 1104–1114, 2005
Conkright MD, Montminy M: CREB: the unindicted cancer co-conspirator. Trends Cell Biol 15: 457–459, 2005
Kinjo K, Sandoval S, Sakamoto KM, Shankar DB: The Role of CREB as a Proto-Oncogene in Hematopoiesis. Cell Cycle 4: 1134–1135, 2005
Kovach SJ, Price JA, Shaw CM, Theodorakis NG, McKillop IH: Role of cyclic-AMP responsive element binding (CREB) proteins in cell proliferation in a rat model of hepatocellular carcinoma. J␣Cell Physiol 206: 411–419, 2006
Beitner-Johnson D, Millhorn DE, Hypoxia induces phosphorylation of the cyclic AMP response element-binding protein by a novel signaling mechanism J Biol Chem 273: 19834–19839, 1998
Taylor CT, Furuta GT, Synnestvedt K, Colgan SP, Phosphorylation-dependent targeting of cAMP response element binding protein to the ubiquitin/proteasome pathway in hypoxia Proc Natl Acad Sci U S A 97: 12091–12096, 2000
Tokunou T, Shibata R, Kai H, Ichiki T, Morisaki T, Fukuyama K, Ono H, Iino N, Masuda S, Shimokawa H, Egashira K, Imaizumi T, Takeshita A, Apoptosis induced by inhibition of cyclic AMP response element-binding protein in vascular smooth muscle cells Circulation 108: 1246–1252, 2003
Knosp E, Steiner E, Kitz K, Matula C: Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 33: 610–617; discussion 617–618, 1993
Funasaka T, Yanagawa T, Hogan V, Raz A, Regulation of phosphoglucose isomerase/autocrine motility factor expression by hypoxia Faseb J 19: 1422–1430, 2005
Hay JG, The potential impact of hypoxia on the success of oncolytic virotherapy Curr Opin Mol Ther 7: 353–358, 2005
Hoogsteen IJ, Peeters WJ, Marres HA, Rijken PF, van den Hoogen FJ, van der Kogel AJ, Kaanders JH: Erythropoietin receptor is not a surrogate marker for tumor hypoxia and does not correlate with survival in head and neck squamous cell carcinomas. Radiother Oncol 76: 213–218, 2005
Maynard MA, Evans AJ, Hosomi T, Hara S, Jewett MA, Ohh M, Human HIF-3alpha4 is a dominant-negative regulator of HIF-1 and is down-regulated in renal cell carcinoma Faseb J 19: 1396–1406, 2005
Mizukami Y, Jo WS, Duerr EM, Gala M, Li J, Zhang X, Zimmer MA, Iliopoulos O, Zukerberg LR, Kohgo Y, Lynch MP, Rueda BR, Chung DC: Induction of interleukin-8 preserves the angiogenic response in HIF-1alpha-deficient colon cancer cells. Nat Med 11: 992–997, 2005
Das B, Yeger H, Tsuchida R, Torkin R, Gee MF, Thorner PS, Shibuya M, Malkin D, Baruchel S, A hypoxia-driven vascular endothelial growth factor/Flt1 autocrine loop interacts with hypoxia-inducible factor-1alpha through mitogen-activated protein kinase/extracellular signal-regulated kinase 1/2 pathway in neuroblastoma Cancer Res 65: 7267–7275, 2005
Economopoulou M, Bdeir K, Cines DB, Fogt F, Bdeir Y, Lubkowski J, Lu W, Preissner KT, Hammes HP, Chavakis T: Inhibition of pathological retinal neovascularization by {alpha}-defensins. Blood 106: 3831–3838, 2005
Lawrentschuk N, Poon AM, Foo SS, Putra LG, Murone C, Davis ID, Bolton DM, Scott AM, Assessing regional hypoxia in human renal tumours using F-fluoromisonidazole positron emission tomography BJU Int 96: 540–546, 2005
Lewis C, Murdoch C, Macrophage responses to hypoxia: implications for tumor progression and anti-cancer therapies. Am J Pathol 167: 627–635, 2005
Ribatti D, Nico B, Spinazzi R, Vacca A, Nussdorfer GG, The role of adrenomedullin in angiogenesis Peptides 26: 1670–1675, 2005
Varlotto J, Stevenson MA, Anemia, tumor hypoxemia, and the cancer patient Int J Radiat Oncol Biol Phys 63: 25–36, 2005
Kristof RA, Aliashkevich AF, Hans V, Haun D, Meyer B, Thees C, Schramm J, The regional oxygen saturation of pituitary adenomas is lower than that of the pituitary gland: microspectrophotometric study with potential clinical implications. Neurosurgery 53: 880–885; discussion 885–886, 2003
Scheithauer BW, Kurtkaya-Yapicier O, Kovacs KT, Young WF Jr., Lloyd RV, Pituitary carcinoma: a clinicopathological review Neurosurgery 56: 1066–1074; discussion 1066–1074, 2005
Viacava P, Gasperi M, Acerbi G, Manetti L, Cecconi E, Bonadio AG, Naccarato AG, Acerbi F, Parenti G, Lupi I, Genovesi M, Martino E: Microvascular density and vascular endothelial growth factor expression in normal pituitary tissue and pituitary adenomas J Endocrinol Invest 26: 23–28, 2003
O’Donoghue JA, Zanzonico P, Pugachev A, Wen B, Smith-Jones P, Cai S, Burnazi E, Finn RD, Burgman P, Ruan S, Lewis JS, Welch MJ, Ling CC, Humm JL, Assessment of regional tumor hypoxia using 18F-fluoromisonidazole and 64Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone) positron emission tomography: Comparative study featuring microPET imaging, Po2 probe measurement, autoradiography, and fluorescent microscopy in the R3327-AT and FaDu rat tumor models Int J Radiat Oncol Biol Phys 61: 1493–1502, 2005
Reischl G, Ehrlichmann W, Bieg C, Solbach C, Kumar P, Wiebe LI, Machulla HJ, Preparation of the hypoxia imaging PET tracer [18F]FAZA: reaction parameters and automation Appl Radiat Isot 62: 897–901, 2005
Yoshida D, Kim K, Yamazaki M and Teramoto A: Expression of Hypoxia-Inducible Factor 1a and Cathepsin D in Pituitary Adenomas. Endocrine Pathol 16: 123–131, 2005
Brennan PA, Mackenzie N, Quintero M, Hypoxia-inducible factor 1alpha in oral cancer J Oral Pathol Med 34: 385–389, 2005
Hayashi M, Sakata M, Takeda T, Tahara M, Yamamoto T, Okamoto Y, Minekawa R, Isobe A, Ohmichi M, Tasaka K, Murata Y: Up-regulation of Met expression through HIF-1{alpha} is involved in trophoblast invasion under low-oxygen tension. Endocrinology 146: 4682–4689, 2005
Theodoropoulos GE, Lazaris AC, Theodoropoulos VE, Papatheodosiou K, Gazouli M, Bramis J, Patsouris E, Panoussopoulos D: Hypoxia, angiogenesis and apoptosis markers in locally advanced rectal cancer. Int J Colorectal Dis 21: 248–257, 2006
Delghandi MP, Johannessen M, Moens U, The cAMP signalling pathway activates CREB through PKA, p38 and MSK1 in NIH 3T3 cells Cell Signal 17: 1343–1351, 2005
Liu X, Sun SQ, Ostrom RS: Fibrotic lung fibroblasts show blunted inhibition by cAMP due to deficient CREB phosphorylation. J␣Pharmacol Exp Ther 315: 678–687, 2005
Peri A, Conforti B, Baglioni-Peri S, Luciani P, Cioppi F, Buci L, Corbetta S, Ballare E, Serio M, Spada A, Expression of cyclic adenosine 3′,5′-monophosphate (cAMP)-responsive element binding protein and inducible-cAMP early repressor genes in growth hormone-secreting pituitary adenomas with or without mutations of the Gsalpha gene J Clin Endocrinol Metab 86: 2111–2117, 2001
Spada A, Arosio M, Bochicchio D, Bazzoni N, Vallar L, Bassetti M, Faglia G, Clinical, biochemical, and morphological correlates in patients bearing growth hormone-secreting pituitary tumors with or without constitutively active adenylyl cyclase J Clin Endocrinol Metab 71: 1421–1426, 1990
Paek KI, Kim SH, Song SH, Choi SW, Koh HS, Youm JY, Kim Y, Clinical significance of Ki-67 labeling index in pituitary macroadenoma J Korean Med Sci 20: 489–494, 2005
Bohinski RJ, Warnick RE, Gaskill-Shipley MF, Zuccarello M, van Loveren HR, Kormos DW, Tew Jr. JM, Intraoperative magnetic resonance imaging to determine the extent of resection of pituitary macroadenomas during transsphenoidal microsurgery Neurosurgery 49: 1133–1143; discussion 1143–1134, 2001
Onizuka M, Tokunaga Y, Shibayama A, Miyazaki H, Computer-assisted neurosurgical navigational system for transsphenoidal surgery–technical note Neurol Med Chir (Tokyo) 41: 565–568; discussion 569, 2001
Pan LX, Chen ZP, Liu YS, Zhao JH, Magnetic resonance imaging and biological markers in pituitary adenomas with invasion of the cavernous sinus space J Neurooncol 74: 71–76, 2005
Knappe UJ, Hagel C, Lisboa BW, Wilczak W, Ludecke DK, Saeger W, Expression of serine proteases and metalloproteinases in human pituitary adenomas and anterior pituitary lobe tissue Acta Neuropathol (Berl) 106: 471–478, 2003
Trouillas J, Daniel L, Guigard MP, Tong S, Gouvernet J, Jouanneau E, Jan M, Perrin G, Fischer G, Tabarin A, Rougon G, Figarella-Branger D: Polysialylated neural cell adhesion molecules expressed in human pituitary tumors and related to extrasellar invasion J Neurosurg 98: 1084–1093, 2003
Acknowledgements
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan (No. 15390445 and No. 17591536), and Grants-in-Aid from the Ministry of Health, Labour and Welfare, Japan, for Cancer Research (17–21).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morimoto, D., Yoshida, D., Noha, M. et al. Phosphorylation of cAMP response element binding protein (CREB) as a marker of hypoxia in pituitary adenoma. J Neurooncol 79, 143–150 (2006). https://doi.org/10.1007/s11060-006-9131-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-006-9131-3