Abstract
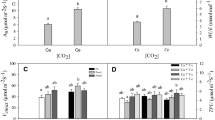
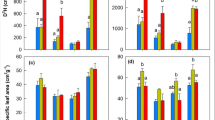
Climate warming has resulted in rapid range shifts of plant species, but it is not well known how species with different natural distribution ranges adapt to increase in temperature through physiological adjustment. We experimentally imposed a 1.8 °C increase of air temperature to the cuttings of two common poplar species Populus yunnanensis and Populus szechuanica naturally growing in southwest China using open-top chambers. Populus yunnanensis is distributed along a narrower elevation range compared with P. szechuanica. We determined some key physiological parameters and plant growth regulator activities during the growing season without soil water limitation. Our results showed that a 1.8 °C increase in air temperature increased shoot growth of P. szechuanica through an extension of its growth period but did not affect the growth of P. yunnanensis. Malondialdehyde content, guaiacol peroxidase activities and abscisic acid content increased while indoleacetic acid content decreased in P. yunnanensis. Our results suggest that the two common poplar species in southwest China should be able to adapt to the moderate increase in temperature projected for future climate. The growth of P. szechuanica may benefit through phenological adjustment but a further increase in temperature may inhibit the growth of P. yunnanensis. For poplar plantation management, selecting species with a wide natural distribution range could provide an adaptive alternative for buffering anthropogenic induced increase in temperature and help in sustaining productivity for the long term.





Similar content being viewed by others
References
Araújo MB, Peterson AT (2012) Uses and misuses of bioclimatic envelope modeling. Ecology 93:1527–1539
Asada K (1992) Ascorbate peroxidase-a hydrogen peroxide-scavenging enzyme in plants. Physiol Plant 85:235–241
Baxter A, Mittler R, Suzuki N (2014) ROS as key players in plant stress signaling. J Exp Bot 65:1229–1240
Beuchamp CH, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Boisvert-Marsh L, Périé C, de Blois S (2014) Shifting with climate? Evidence for recent changes in tree species distribution at high latitudes. Ecosphere 5:1–33
Breshears DD, Huxman TE, Adams HD, Zou CB, Davison JE (2008) Vegetation synchronously leans upslope as climate warms. PNAS 105:11591–11592
Bronson DR, Gower ST, Tanner M, Vanherk I (2009) Effect of ecosystem warming on boreal black spruce bud burst and shoot growth. Glob Change Biol 15:1534–1543
Case MJ, Lawler JJ (2017) Integrating mechanistic and empirical model projections to assess climate impacts on tree species distributions in northwestern North America. Glob Change Biol 23:2005–2015
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. Methods Enzymol 2:764–775
Chen IC, Hill JK, Ohlemüller R, Roy DB, Thomas CD (2011) Rapid range shifts of species associated with high levels of climate warming. Science 333:1024–1026
Chmielewski F, Rötzer T (2001) Response of tree phenology to climate change across Europe. Agr For Meteorol 108:101–112
Contran N, Günthardt-Goerg MS, Kuster TM, Cerana R, Potassium Crosti, Paoletti E (2013) Physiological and biochemical responses of Quercus pubescens to air warming and drought on acidic and calcareous soils. Plant Biol 15:157–168
Daeter W, Hartung W (1995) Stress-dependent redistribution of abscisic acid (ABA) in Hordeum vulgare L. leaves: the role of epidermal ABA metabolism, the tonoplastic transport and the cuticle. Plant Cell Environ 18:1367–1376
Dodd IC, Davies WJ (2004) Hormones and the regulation of water balance. In: Davis PJ (ed) Plant hormones: biosynthesis, signal transduction, action!, 3rd edn. Kluwer Academic Publishers, Dordrecht, pp 493–512
Drake JE, Aspinwall MJ, Pfautsch S et al (2015) The capacity to cope with climate warming declines from temperate to tropical latitudes in two widely distributed Eucalyptus species. Glob Change Biol 21:459–472
Duan BL, Zhang XL, Li YP, Li L, Korpelainen H, Li CY (2013) Plastic responses of Poplulus yunnanensis and Abies faxoniana to elevated atmospheric CO2 and warming. For Ecol Manag 296:33–40
Duan BL, Dong TF, Zhang XL, Zhang YB, Chen J (2014) Ecophysiological responses of two dominant subalpine tree species Betula albo-sinensis and Abies faxoniana to intra- and interspecific competition under elevated temperature. For Ecol Manag 323:20–27
Elstner EF (1982) Oxygen activation and oxygen toxicity. Annu Rev Plant Physiol 33:73–96
Finkelstein RR, Gampala SS, Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell 14(Suppl):S15–S45
Gunderson CA, Norby RJ, Wullschleger SD (2000) Acclimation of photosynthesis and respiration to simulated climatic warming in northern and southern populations of Acer saccharum: laboratory and field evidence. Tree Physiol 20:87–96
Halliwell B, Gutteridge JMC (1989) Free radicals in biology and medicine, 2nd edn. Clarendon Press, New York
Heskel M, Greaves H, Kornfeld A et al (2013) Differential physiological responses to environmental change promote woody shrub expansion. Ecol Evol 3:1149–1162
Hyvönen R, Ågren GI, Linder S et al (2007) The likely impact of elevated CO2, nitrogen deposition, increased temperature and management on carbon sequestration in temperate and boreal forest ecosystems: a literature review. New Phytol 173:463–480
Intergovernmental Panel on Climate Change (IPCC) (2007) The physical sciences Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change, Geneva
Li FF, Zhan D, Xu LX, Han LB (2014a) Antioxidant and hormone responses to heat stress in two Kentucky bluegrass cultivars contrasting in heat tolerance. J Am Soc Hortic Sci 139:587–596
Li X, Yang YQ, Sun XD, Lin HM et al (2014b) Comparative physiological and proteomic analyses of poplar (Populus yunnanensis) plantlets exposed to high temperature and drought. PLoS One 9:e107605
Menzel A, Fabian P (1999) Growing season extended in Europe. Nature 397:659
Molau U, Mølgaard P (eds) (1996) International Tundra Experiment (ITEX) Manual, 2nd edn. Danish Polar Center, Copenhagen, Denmark
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Biol 49:249–279
Noctor G, Gomez L, Vanacker H, Foyer CH (2002) Interactions between biosynthesis, compartmentation, and transport in the control of glutathione homeostasis and signaling. J Exp Bot 53(2002):1283–1304
Okuda T, Matsuda Y, Yamanaka A, Sagisaka S (1991) Abrupt increases in the level of hydrogen peroxide in leaves of winter wheat is caused by cold treatment. Plant Physiol 97:1265–1267
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Ann Rev Ecol Syst 37:637–669
Pearson RG, Dawson TP (2003) Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Glob Ecol Biogeogr 12:361–371
Pence VC, Caruso JL (1986) Auxin and cytokinin levels in selected and temperature-induced morphologically distinct lines of tobacco crown gall tumors. Plant Sci 46:233–237
Polle A, Klein T, Kettner C (2013) Impact of cadmium on young plants of Populus euphratica and P. × canescens, two poplar species that differ in stress tolerance. New For 44:13–22
Sage RF, Kubien DS (2007) The temperature response of C3 and C4 photosynthesis. Plant Cell Environ 30:1086–1106
Schieber M, Chandel NS (2014) ROS function in redox signaling and oxidative stress. Curr Biol 24:R453–R462
Shi SL, Wang YC, Zhou JH, Zhou HB (2011) Endogenous hormone contents and their habitat differentia of Reaumuria trigyna and R. soongorica in different salt habitats. Chin J Appl Ecol 22:350–356
Somero GN (2010) The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J Exp Biol 213:912–920
Song XL, Wang YH, Lv XM (2016) Responses of plant biomass, photosynthesis and lipid peroxidation to warming and precipitation change in two dominant species (Stipa grandis and Leymus chinensis) from North China Grasslands. Ecol Evol 6(6):1871–1882
Strivastava LM (2002) Plant growth and development: hormones and environment. Academic Press, San Diego
Tang RS, Zheng JC, Jin ZQ et al (2008) Possible correlation between high temperature-induced floret sterility and endogenous levels of IAA, GAs and ABA in rice (Oryza sativa L.). Plant Growth Regul 54:37–43
Templer PH, Phillips NG, Ellison AM, Pelini SL (2016) Ecosystem warming increases sap flow rates of northern red oak trees. Ecosphere 7:e01221. https://doi.org/10.1002/ecs2.1221
Wang Z, Fang ZF (1984) Flora Reipublicae Popularis Sinicae, Salicaceae of China, vol 20(2). Science Press, Beijing
Wang LJ, Huang WD, Liu YP, Zhan JC (2005) Changes in salicylic and abscisic acid contents during heat treatment and their effect on thermotolerance of grape plants. Russ J Plant Physiol 52:516–520
Wang JC, Duan BL, Zhang YB (2012) Effects of experimental warming on growth, biomass allocation, and needle chemistry of Abies faxoniana in even-aged monospecific stands. Plant Ecol 213:47–55
Way DA, Oren R (2010) Differential responses to changes in growth temperature between trees from different functional groups and biomes: a review and synthesis of data. Tree Physiol 30:669–688
Xiong LM, Schumaker KS, Zhu JK (2002) Cell signaling during cold, drought, and salt stress. Plant Cell 14:S165–S183
Yamori W, Noguchi K, Terashima I (2005) Temperature acclimation of photosynthesis in spinach leaves: analyses of photosynthetic components and temperature dependencies of photosynthetic partial reactions. Plant Cell Environ 28:536–547
Yin HJ, Liu Q, Lai T (2008) Warming effects on growth and physiology in the seedlings of the two conifers Picea asperata and Abies faxoniana under two contrasting light conditions. Ecol Res 23:459–469
Zacchini M, Rea E, Tullio M, de Agazio M (2003) Increased antioxidative capacity in maize calli during and after oxidative stress induced by a long lead treatment. Plant Physiol Biochem 41:49–54
Zhu H (2015) Geographical patterns of Yunnan seed plants may be influenced by the clockwise rotation of the Simao-Indochina geoblock. Front Earth Sci 3:53. https://doi.org/10.3389/feart.2015.00053
Acknowledgements
The research was supported by the National Natural Science Foundation of China (No. 31260167). We thank Elaine Stebler and one anonymous reviewer for their insightful suggestions and editorial comments. We thank the financial support of the China Scholarship Council which supported Dr. Ren in data analysis and manuscript preparation at Oklahoma State University, Stillwater, Oklahoma.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ren, J., Dai, W., Yang, C. et al. Physiological regulation of poplar species to experimental warming differs between species with contrasting elevation ranges. New Forests 49, 329–340 (2018). https://doi.org/10.1007/s11056-017-9622-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-017-9622-4