Abstract
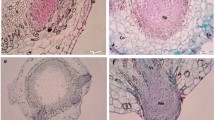
Low amenability of the eucalypt, Corymbia torelliana × C. citriodora, to vegetative propagation has limited its establishment in plantations. This study determined whether rooting capacity and cutting vigour varied along the central shoot of Corymbia seedlings, and whether these differences are related to stem development and hormone concentrations. Dual-node cuttings were harvested from five positions (nodes 1/2, 3/4, 5/6, 7/8 and 9/10) along the seedling shoot (S1), and cuttings were treated with one of four levels of indole-3-butyric acid (IBA). Root formation, root and shoot growth, stem anatomy, and the concentrations of indole-3-acetic (IAA) and abscisic acid (ABA) were assessed at each shoot position. The central node of the rooted cuttings was harvested for fresh cuttings to observe whether maturation emerged in ramets (S2) from the different ortet positions. Rooting and vigour were highest from the most-apical seedling nodes (7/8 and 9/10 in S1), which had less lignification and sclerenchyma development than more-basal nodes (1/2, 3/4 and 5/6). IAA and ABA concentrations differed little between the seedling nodes. In contrast, cuttings from the ramets (in S2) had similar rooting, vigour, lignification and sclerification to each other, but there were large differences in IAA and ABA concentrations depending on their previous point-of-origin in the seedling. High doses of IBA reduced rooting. Rooting and vigour were related to lignification and sclerenchyma development rather than IAA or ABA concentrations. Mass vegetative propagation of C. torelliana × C. citriodora is achievable because mean rooting percentages in S1 and S2 were 61 and 68 %, respectively, using cuttings from small seedling ortets and their ramets.







Similar content being viewed by others
References
Abu-Abied M, Szwerdszarf D, Mordehaev I, Levy A, Rogovoy O, Belausov E, Yaniv Y, Uliel S, Katzenellenbogen M, Riov J, Ophir R, Sadot E (2012) Microarray analysis revealed upregulation of nitrate reductase in juvenile cuttings of Eucalyptus grandis, which correlated with increased nitric oxide production and adventitious root formation. Plant J 71:787–799
Ahkami AH, Melzer M, Ghaffari M, Pollmann S, Javid MG, Shahinnia F, Hajirezaei MR, Druege U (2013) Distribution of indole-3-acetic acid in Petunia hybrids shoot tip cuttings and relationship between auxin transport, carbohydrate metabolism and adventitious root formation. Planta 238:499–517
Amissah JN, Paolillo DJ Jr, Bassuk N (2008) Adventitious root formation in stem cuttings of Quercus bicolor and Quercus macrocarpa and its relationship to stem anatomy. J Am Soc Hortic Sci 133:479–486
Brondani GE, Grossi F, Wendling I, Dutra LF, Araujo MA (2010) Aplicação de IBA para o enraizamento de miniestacas de Eucalyptus benthamii Maiden Cambage x Eucalyptus dunnii Maiden. Acta Sci Agron 32:667–674
Brondani GE, Wendling I, Brondani AE, Araujo MA, Silva ALL, Gonçalves AN (2012) Dynamics of adventitious rooting in mini-cuttings of Eucalyptus benthamii x Eucalyptus dunnii. Acta Sci Agron 34:169–178
Chen CW, Yang YW, Lur HS, Tsai YG, Chang MC (2006) A novel function of abscisic acid in the regulation of rice (Oryza sativa) root growth and development. Plant Cell Physiol 47:1–13
Costa CT, de Almeida MR, Ruedell CM, Schwambach J, Maraschin FS, Fett-Neto AG (2013) When stress and development go hand in hand: main hormonal controls of adventitious rooting in cuttings. Front Plant Sci 4:1–19
Davies PJ (2010) The plant hormones: their nature, occurrence, and functions. In: Davies PJ (ed) plant hormones. Springer, Netherlands, pp 1–15
Dickinson GR, Wallace HM, Lee DJ (2013) Reciprocal and advanced generation hybrids between Corymbia citriodora and C. torelliana: forestry breeding and the risk of gene flow. Ann For Sci 70:1–10
Ford YY, Bonham EC, Cameron RWF, Blake PS, Judd HL, Harrison-Murray RS (2002) Adventitious rooting: examining the role of auxin in an easy-and a difficult-to-root plant. Plant Growth Reg 36:149–159
George EF (1993) Plant propagation by tissue culture. Part 1. The technology. Exegetics Ltd, Edington, UK
Hartmann HT, Kester DE, Davies Junior FT, Geneve RL (2011) Plant propagation: principles and practices. Prentice-Hall, New Jersey
Hopkins WG, Huner NPA (2004) Introduction to plant physiology. Wiley, London
Hung CD, Trueman SJ (2011) Topophysic effects differ between node and organogenic cultures of the eucalypt Corymbia torelliana × C. citriodora. Plant Cell, Tissue Organ Cult 104:69–77
Hung CD, Trueman SJ (2012) Alginate encapsulation of shoot tips and nodal segments for short-term storage and distribution of the eucalypt Corymbia torelliana × C. citriodora. Acta Physiol Plant 34:117–128
Husen A (2012) Changes of soluble sugars and enzymatic activities during adventitious rooting in cuttings of Grewia optiva as affected by age of donor plants and auxin treatments. Am J Plant Physiol 7:1–16
Husen A, Pal M (2007) Effect of branch position and auxin treatment on clonal propagation of Tectona grandis Linn. f. New For 34:223–233
Kelen M, Ozkan G (2003) Relationships between rooting ability and changes of endogenous IAA and ABA during rooting of hardwood cuttings of some grapevine rootstocks. Eur J Hortic Sci 68:8–13
Kelen M, Demiralay EC, Sen S, Ozkan G (2004) Separation of abscisic acid, indole-3-acetic acid, gibberellic acid in 99 R (Vitis berlandieri × Vitis rupestris) and rose oil (Rosa damascena Mill.) by reversed phase liquid chromatography. Turk J Chem 28:603–610
Kilkenny AJ, Wallace HM, Walton DA, Adkins MF, Trueman SJ (2012) Improved root formation in eucalyptus cuttings following combined auxin and anti-ethylene treatments. J Plant Sci 7:138–153
Lee DJ (2007) Achievements in forest tree genetic improvement in Australia and New Zealand 2: development of Corymbia species and hybrids for plantations in eastern Australia. Aust For 70:11–16
Mason WL, Menzies MI, Biggin P (2002) A comparison of hedging and repeated cutting cycles for propagating clones of Sitka spruce. Forest 75:149–162
Materán ME, Fernández M, Valenzuela S, Sáez K, Seemann P, Sánchez-Olate M, Ríos D (2009) Abscisic acid and 3-indolacetic acid levels during the reinvigoration process of Pinus radiata D. Don adult material. Plant Growth Regul 59:171–177
Maynard BK, Bassuk NL (1996) Effects of stock plant etiolation, shading, banding, and shoot development on histology and cutting propagation of Carpinus betulus L. fastigiata. J Am Soc Hortic Sci 121:853–860
McMahon TV, Hung CD, Trueman SJ (2014) Clonal maturation of Corymbia torelliana × C. citriodora is delayed by minimal-growth storage. Aust For 77:9–14
Mitchell RG, Zwolinski J, Jones NB (2004) A review on the effects of donor maturation on rooting and field performance of conifer cuttings. South Afr For J 201:53–63
Mwange WN, Hou HW, Cui KM (2003) Relationship between endogenous indole-3-acetic acid and abscisic acid changes and bark recovery in Eucommia ulmoides Oliv. after girding. J Exp Bot 54:1899–1907
Negishi N, Nakahama K, Urata N, Kojima M, Sakakibara H, Kawaoka A (2014) Hormone level analysis on adventitious root formation in Eucalyptus globulus. New For 45:577–587
Osterc G, Štampar F (2011) Differences in endo/exogenous auxin profile in cuttings of different physiological ages. J Plant Physiol 168:2088–2092
Osterc G, Štefančič M, Štampar F (2009) Juvenile stockplant material enhances root development through higher endogenous auxin level. Acta Physiol Plant 31:899–903
Pacholczak A, Szydło W, Łukaszewska A (2006) The effect of shading of stock plants on rhizogenesis in stem cuttings of Berberis thunbergii ‘Red Rocket’. Acta Physiol Plant 28:567–575
Paton DM, Willing RR, Nicholls W, Pryor LD (1970) Rooting of stem cuttings of Eucalyptus: a rooting inhibitor in adult tissue. Aust J Bot 18:175–183
Peer WA, Blakeslee JJ, Yang H, Murphy AS (2011) Seven things we think we know about auxin transport. Mol Plant 4:487–504
Perry F, Trueman SJ (1999) Cutting propagation of Victorian smoke bush, Conospermum mitchellii (Proteaceae). S Afr J Bot 65:243–244
Pijut PM, Wowste KE, Michler CH (2011) Promotion of adventitious root formation of difficult-to-root hardwood tree species. Hortic Rev 38:213–251
Pohio KE, Wallace HM, Peters RF, Smith TE, Trueman SJ (2005) Cuttings of Wollemi pine tolerate moderate photoinhibition and remain highly capable of root formation. Trees Struct Funct 19:587–595
Rasmussen A, Hunt MA (2010) Ageing delays the cellular stages of adventitious root formation in pine. Aust For 73:41–46
Rinne P, Tuominen H, Sundberg B (1993) Growth patterns and endogenous indole-3-acetic acid concentrations in current-year coppice shoots and seedlings of two Betula species. Physiol Plant 88:403–412
Shepherd M, Pomroy P, Dieters M, Lee D (2007) Genetic control of propagation traits in a single Corymbia torelliana × Corymbia variegata family. Can J For Res 37:2563–2574
Shkolnik-Inbar D, Bar-Zvi D (2010) ABI4 mediates abscisic acid and cytokinin inhibition of lateral root formation by reducing polar auxin transport in Arabidopsis. Plant Cell 22:3560–3573
Stewart JL, Nemhauser JL (2010) Do trees grow on money? Auxin as the currency of the cellular economy. Cold Spring Harb Perspect Biol 2:2–14
Trueman SJ (2006) Clonal propagation and storage of subtropical pines in Queensland, Australia. South Afr For J 208:49–52
Trueman SJ, Adkins MF (2013) Effect of aminoethoxyvinylglycine and 1-methylcyclopropene on leaf abscission and root formation in Corymbia and Eucalyptus cuttings. Sci Hortic 161:1–7
Trueman SJ, Peters RF (2006) Propagation of Wollemi pine from tip cuttings and lower segment cuttings does not require rooting hormones. Sci Hortic 109:394–397
Trueman SJ, Richardson DM (2008) Relationships between indole-3-butyric acid, photoinhibition and adventitious rooting of Corymbia torelliana, C. citriodora and F1 hybrid cuttings. Tree For Sci Biotechnol 2:26–33
Tsipouridis C, Thomidis T, Bladenopoulou S (2006) Rhizogenesis of GF677, Early Crest, May Crest and Arm King stem cuttings during the year in relation to carbohydrate and natural hormone content. Sci Hortic 108:200–204
von Aderkas P, Bonga JM (2000) Influencing micropropagation and somatic embryogenesis in mature trees by manipulation of phase change, stress and culture environment. Tree Physiol 20:921–928
Weigel U, Horn W, Hock B (1984) Endogenous auxin levels in terminal stem cuttings of Chrysanthemum morifolium during adventitious rooting. Physiol Plant 61:422–428
Wendling I, Xavier A (2005) Influência do ácido indolbutírico e da miniestaquia seriada no enraizamento e vigor de miniestacas de clones de Eucalyptus grandis. Rev Árv 29:921–930
Wendling I, Trueman SJ, Xavier A (2014a) Maturation and related aspects in clonal forestry—Part I: concepts, regulation and consequences of phase change. New For 45:449–471
Wendling I, Trueman SJ, Xavier A (2014b) Maturation and related aspects in clonal forestry—part II: reinvigoration, rejuvenation and juvenility maintenance. New For 45:473–486
White J, Lovell PH (1984) Anatomical changes which occur in cuttings of Agathis australis (D. Don) Lindl 2. The initiation of root primordia and early root development. Ann Bot 54:633–645
Yamaguchi M, Sharp RE (2010) Complexity and coordination of root growth at low water potentials: recent advances from transcriptomic and proteomic analyses. Plant, Cell Environ 33:590–603
Acknowledgments
We thank Tracey McMahon and Pip Bryant for assistance and David Lee (Agri-Science Queensland) for providing seeds. This work was supported by the Brazilian Agricultural Research Corporation (Embrapa) and the Queensland Plantation Hardwoods Research Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wendling, I., Brooks, P.R. & Trueman, S.J. Topophysis in Corymbia torelliana × C. citriodora seedlings: adventitious rooting capacity, stem anatomy, and auxin and abscisic acid concentrations. New Forests 46, 107–120 (2015). https://doi.org/10.1007/s11056-014-9451-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-014-9451-7