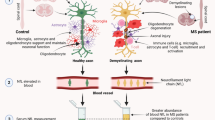
Multiple sclerosis (MS) is a chronic autoimmune disease affecting the spinal cord and brain. Detection of the disease at its initial stages is a difficult task as the causes and mechanisms of the manifestations of the disease remain unclear. Diagnosis of MS is a complex process. Studies of the molecular mechanisms of the disease and the search for biomarkers are among the key directions in the diagnosis of the disease. This review addresses potential biomarkers for multiple sclerosis detected in the cerebrospinal fluid.
Similar content being viewed by others
References
M. M. Goldenberg, “Multiple sclerosis review,” Pharmac. Ther., 37, No. 3, 175–184 (2012).
I. Loma and R. Heyman, “Multiple sclerosis: pathogenesis and treatment,” Curr. Neuropharmacol., 9, No. 3, 409–416 (2011), https://doi.org/10.2174/157015911796557911.
A. Katdare and M. Ursekar, “Systematic imaging review: Multiple sclerosis,” Ann. Ind. Acad. Neurol., 18, Suppl. 1, 24–29 (2015), https://doi.org/10.4103/0972-2327.164821.
B. R. Sajja, J. S. Wolinsky, and P. A. Narayana, “Proton magnetic resonance spectroscopy in multiple sclerosis,” Neuroimaging Clin. N. Am., 19, No. 1, 45–58 (2009), https://doi.org/10.1016/j.nic.2008.08.002.
P. A. Gourraud, J. P. McElroy, S. J. Caillier, et al., “Aggregation of multiple sclerosis genetic risk variants in multiple and single case families,” Ann. Neurol., 69, No. 1, 65–74 (2011), https://doi.org/10.1002/ana.22323.
P. Sundström, P. Juto, G. Wadell, et al., “An altered immune response to Epstein–Barr virus in multiple sclerosis: A prospective study,” Neurology, 62, 2277–2282 (2004), https://doi.org/10.1212/01.WNL.0000130496.51156.D7.
J. M. Nielsen, T. Korteweg, F. Barkhof, et al., “Overdiagnosis of multiple sclerosis and magnetic resonance imaging criteria,” Ann. Neurol., 58, 781–783 (2005), https://doi.org/10.1002/ana.20632.
D. Miller, F. Barkhof, X. Montalban, et al., “Clinically isolated syndromes suggestive of multiple sclerosis. Part I: Natural history, pathogenesis, diagnosis, and prognosis,” Lancet Neurol., 4, 281–288 (2005), https://doi.org/10.1016/S1474-4422(05)70071-5.
H. Lassmann, W. Bruck, and C. F. Lucchinetti, “The immunopathology of multiple sclerosis: an overview,” Brain Pathol., 17, No. 2, 210–218 (2007), https://doi.org/10.1111/j.1750-3639.2007.00064.x.
C. H. Polman, S. C. Reingold, B. Banwell, et al., “Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria,” Ann. Neurol., 69, No. 2, 292–302 (2011), https://doi.org/10.1002/ana.22366.
A. J. Thompson, B. L. Banwell, F. Barkhof, et al., “Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria,” Lancet Neurol., 17, No. 2, 162–173 (2018), https://doi.org/10.1016/S1474-4422(17)30470-2.
R. Dobson, S. Ramagopalan, A. Davis, and G. Giovannoni, “Cerebrospinal fluid oligoclonal bands in multiple sclerosis and clinically isolated syndromes: a meta-analysis of prevalence, prognosis and effect of latitude,” J Neurol. Neurosurg. Psychiatry, 84, No. 8, 909–914 (2013), https://doi.org/10.1136/jnnp-2012-304695.
A. Petzold, “Intrathecal oligoclonal IgG synthesis in multiple sclerosis,” J. Neuroimmunol., 262, No. 1–2, 1–10 (2013), https://doi.org/10.1016/j.jneuroim.2013.06.014.
I. Nakashima, K. Fujihara, S. Sato, and Y. Itoyama, “Oligoclonal IgG bands in Japanese patients with multiple sclerosis. A comparative study between isoelectric focusing with IgG immunofi xation and high-resolution agarose gel electrophoresis,” J. Neuroimmunol., 159, 133–136 (2005), https://doi.org/10.1016/j.jneuroim.2004.09.011.
A. S. Fortini, E. L. Sanders, B. G. Weinshenker, and J. A. Katzmann, “Cerebrospinal fluid oligoclonal band in the diagnosis of multiple sclerosis,” Am. J. Clin. Pathol., 120, 672–675 (2003), https://doi.org/10.1309/EM7K-CQR4-GLMH-RCX4.
H. Link and Y.-M. Huang, “Oligoclonal bands in multiple sclerosis cerebrospinal fluid: an update on methodology and clinical usefulness,” J. Neuroimmunol., 180, 17–28 (2006), https://doi.org/10.1016/j.jneuroim.2006.07.006.
R. Tomioka and M. Matsui, “Biomarkers for multiple sclerosis,” Internal Medicine, 53, 361–365 (2014), https://doi.org/10.2169/internalmedicine.53.1246.
M. Thangarajh, J. Gomez-Rial, A. K. Hedström, et al., “Lipidspecific immunoglobulin M in CSF predicts adverse long-term out-come in multiple sclerosis,” Mult. Scler., 14, No. 9, 1208–1213 (2008), https://doi.org/10.1177/1352458508095729.
L. M. Villar, M. C. Sádaba, E. Roldán, et al., “Intrathecal synthesis of oligoclonal IgM against myelin lipids predicts an aggressive disease course in MS,” J. Clin. Invest., 115, No. 1, 187–194 (2005), https://doi.org/10.1172/JCI200522833.
J. C. Alvarez-Cermeno, F. J. Munoz-Negrete, L. Costa-Frossard, et al., “Intrathecal lipid-specific oligoclonal IgM synthesis associates with retinal axonal loss in multiple sclerosis,” J. Neurol. Sci., 360, 41–44 (2016), https://doi.org/10.1016/j.jns.2015.11.030.
L. M. Villar, C. Picón, L. Costa-Frossard, et al., “Cerebrospinal fluid immunological biomarkers associated with axonal damage in multiple sclerosis,” Eur. J. Neurol., 22, No. 8, 1169–1175 (2015), https://doi.org/10.1111/ene.12579.
M. C. Sádaba, J. Tzartos, C. Paino, et al., “Axonal and oligodendrocytelocalized IgM and IgG deposits in MS lesions,” J. Neuroimmunol., 247, No. 1–2, 86–94 (2012), https://10.1016/j.jneuroim.2012.03.020.
E. Cantó, M. Tintoré, L. M. Villar, et al., “Chitinase 3-like 1: prognostic biomarker in clinically isolated syndromes,” Brain, 138, No. 4, 918–931 (2015), https://doi.org10.1093/brain/awv017.
M. Kanneganti, A. Kamba, and E. Mizoguchi, “Role of chitotriosidase (chitinase 1) under normal and disease conditions,” J. Epithel. Biol. Pharmacol., 5, 1–9 (2012).
G. Hinsinger, N. Galéotti, N. Nabholz, et al., “Chitinase 3-like proteins as diagnostic and prognostic biomarkers of multiple sclerosis,” Mult. Scler., 21, No. 10, 1251–1261 (2015), https://doi.org/10.1177/1352458514561906.
C. G. Lee, C. A. Da Silva, C. S. Dela Cruz, et al., “Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury,” Annu. Rev. Physiol., 73, 479–501 (2011), https://doi.org/10.1146/annurev-physiol-012110-142250.
D. Bonneh-Barkay, G. Wang, A. Starkey, et al., “In vivo CHI3L1 (YKL-40) expression in astrocytes in acute and chronic neurological diseases,” J. Neuroinflammation, 7, 34 (2010), https://doi.org/10.1186/1742-2094-7-34.
M. A. Martinez, B. Olsson, L. Bau, et al., “Glial and neuronal markers in cerebrospinal fluid predict progression in multiple sclerosis,” Mult. Scler., 21, No. 5, 550–561 (2015), https://doi.org/10.1177/1352458514549397.
S. Modvig, M. Degn, B. Sander, et al., “Cerebrospinal fluid neurofi lament light chain levels predict visual outcome after optic neuritis,” Mult. Scler., 22, No. 5, 590–598 (2016), https://doi.org/10.1177/1352458515599074.
E. Borràs, E. Canto, M. Choi, et al., “Protein-based classifi er to predict conversion from clinically isolated syndrome to multiple sclerosis,” Mol. Cell. Proteomics, 15, No. 1, 318–328 (2016), https://doi.org/10.1074/mcp.M115.053256.
M. Mollgaard, M. Degn, F. Sellebjerg, et al., “Cerebrospinal fluid chitinase-3-like 2 and chitotriosidase are potential prognostic biomarkers in early multiple sclerosis,” Eur. J. Neurol., 23, No. 5, 898–905 (2016), https://doi.org/10.1111/ene.12960.
J. Kuhle, D. Leppert, A. Petzold, et al., “Neurofi lament heavy chain in CSF correlates with relapses and disability in multiple sclerosis,” Neurology, 76, No. 14, 1206–1213 (2011), https://doi.org/10.1212/WNL.0b013e31821432ff.
C. E. Teunissen, E. Iacobaeus, M. Khademi, et al., “Combination of CSF N-acetylaspartate and neurofi laments in multiple sclerosis,” Neurology, 72, No. 15, 1322–1329 (2009), https://doi.org/10.1212/WNL.0b013e3181a0fe3f.
J. Salzer, A. Svenningsson, and P. Sundstrom, “Neurofi lament light as a prognostic marker in multiple sclerosis,” Mult. Scler., 16, No. 3, 287–292 (2010), https://doi.org/10.1177/1352458509359725.
C. Matute-Blanch, L. M. Villar, J. C. Álvarez-Cermeño, et al., “Neurofi lament light chain and oligoclonal bands are prognostic biomarkers in radiologically isolated syndrome,” Brain, 141, No. 4, 1085–1093 (2018), https://doi.org/10.1093/brain/awy021.
A. Bhan, C. Jacobsen, K. M. Myhr, et al., “Neurofi laments and 10-year follow-up in multiple sclerosis,” Mult. Scler., 24, No. 10, 1301–1307 (2018), https://doi.org/10.1177/1352458518782005.
L. Cai and J. Huang, “Neurofi lament light chain as a biological marker for multiple sclerosis: a meta-analysis study,” Neuropsychiatr. Dis. Treat., 14, 2241–2254 (2018), https://doi.org/10.2147/NDT.S173280.
A. Petzold, M. D. Steenwijk, J. M. Eikelenboom, et al., “Elevated CSF neurofi lament proteins predict brain atrophy: a 15-year follow-up study,” Mult. Scler., 22, No. 9, 1154–1162 (2016), https://doi.org/10.1177/1352458516645206.
A. Petzold, “The prognostic value of CSF neurofi laments in multiple sclerosis at 15-year follow-up,” J Neurol. Neurosurg. Psychiatry, 86, No. 12, 1388–1390 (2015), https://doi.org/10.1136/jnnp-2014-309827.
C. M. Jacque, C. Vinner, M. Kujas, et al., “Determination of glial fibrillary acidic protein (GFAP) in human brain tumors,” J. Neurol. Sci., 35, 147–155 (1978), https://doi.org/10.1016/0022-510X(78)90107-7.
U. Roessmann, M. E. Velasco, S. D. Sindely, and P. Gambetti, “Glial fibrillary acidic protein (GFAP) in ependymal cells during development. An immunocytochemical study,” Brain Res., 200, 13–21 (1980), https://doi.org/10.1016/0006-8993(80)91090-2.
M. A. Martínez, B. Olsson, L. Bau, et al., “Glial and neuronal markers in cerebrospinal fluid predict progression in multiple sclerosis,” Mult. Scler., 21, No. 5, 550–561 (2015); https://doi.org/10.1177/1352458514549397.
M. Axelsson, C. Malmeström, S. Nilsson, et al., “Glial fi brillary acidic protein: a potential biomarker for progression in multiple sclerosis,” J. Neurol., 258, No. 5, 882–888 (2011), https://doi.org/10.1007/s00415-010-5863-2.
Y. K. Semra, O. A. Seidi, and M. K. Sharief, “Heightened intrathecal release of axonal cytoskeletal proteins in multiple sclerosis is associated with progressive disease and clinical disability,” J. Neuroimmunol., 122, No. 1–2, 132–139 (2002), https://doi.org/10.1007/s10072-012-0974-4.
R. Madeddu, C. Farace, P. Tolu, et al., “Cytoskeletal proteins in the cerebrospinal fluid as biomarker of multiple sclerosis,” Neurol. Sci., 34, No. 2, 181–186 (2013), https://doi.org/10.1007/s10072-012-0974-4.
J. G. Cyster, “Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs,” Annu. Rev. Immunol., 23, 127–159 (2005), https://doi.org/10.1146/annurev.immunol.23.021704.115628.
T. Okada, and J. G. Cyster, “B cell migration and interactions in the early phase of antibody responses,” Curr. Opin. Immunol., 18, 278–285 (2006), https://doi.org/10.1016/j.coi.2006.02.005.
M. Khademi, I. Kockum, M. L. Andersson, et al., “Cerebrospinal fluid CXCL13 in multiple sclerosis: A suggestive prognostic marker for the disease course,” Mult. Scler., 17, 335–343 (2011), https://doi.org/10.1177/1352458510389102.
J. Brettschneider, A. Czerwoniak, M. Senel, et al., “The chemokine CXCL13 is a prognostic marker in clinically isolated syndrome (CIS),” PLoS One, 5, No. 8, e11986 (2010), https://doi.org/10.1371/journal.pone.0011986.
F. Mashayekhi, Z. Salehi, and H. R. Jamalzadeh, “Quantitative analysis of cerebrospinal fluid brain derived neurotrophic factor in the patients with multiple sclerosis,” Acta Medica (Hradec Kralove), 55, No. 2, 83–86 (2012), https://doi.org10.14712/18059694.2015.60.
T. Khaibullin, V. Ivanova, E. Martynova, et al., “Elevated levels of proinflammatory cytokines in cerebrospinal fluid of multiple sclerosis patients,” Front. Immunol., 8, 531 (2017), https://doi.org/10.3389/fimmu.2017.00531.
J. Losy, P. Iwanowski, E. Kaufman, and M. Wójcicka, “CXCL13 CSF level inversely correlates with duration of disease in primary progressive multiple sclerosis,” J. Med. Sci., 85, No. 4, 298–300 (2016), https://doi.org/10.20883/jms.2016.169.
M. Komori, A. Blake, M. Greenwood, et al., “Cerebrospinal fluid markers reveal intrathecal inflammation in progressive multiple sclerosis,” Ann. Neurol., 78, No. 1, 3–20 (2015), https://doi.org/10.1002/ana.24408.
M. Stilund, A. K. Reuschlein, T. Christensen, et al., “Soluble CD163 as a marker of macrophage activity in newly diagnosed patients with multiple sclerosis,” PLoS One, 9, No. 6, e98588 (2014), https://doi.org/10.1371/journal.pone.0098588.
A. Ohrfelt, M. Axelsson, C. Malmeström, et al., “Soluble TREM-2 in cerebrospinal fluid from patients with multiple sclerosis treated with natalizumab or mitoxantrone,” Mult. Scler., 22, No. 12, 1587–1595 (2016), https://doi.org/10.1177/1352458515624558.
G. Hassan-Smith, L. Durant, A. Tsentemeidou, et al., “High sensitivity and specificity of elevated cerebrospinal fluid kappa free light chains in suspected multiple sclerosis,” J. Neuroimmunol., 276, No. 1–2, 175–1759 (2014), https://doi.org/10.1016/j.jneuroim.2014.08.003.
L. M. Villar, M. Espiño, L. Costa-Frossard, et al., “High levels of cerebrospinal fluid free kappa chains predict conversion to multiple sclerosis,” Clin. Chim. Acta, 413, No. 23–24, 1813–1816 (2012), https://doi.org/10.1016/j.cca.2012.07.007.
P. Menéndez-Valladares, M. I. García-Sánchez, M. Adorna Martínez, et al., “Validation and meta-analysis of kappa index biomarker in multiple sclerosis diagnosis,” Autoimmun. Rev. (2018); pii:1568-9972(18)30259-3, https://doi.org/10.1016/j.autrev.2018.07.010.
M. Christiansen, M. C. Gjelstrup, M. Stilund, et al., “Cerebrospinal fluid free kappa light chains and kappa index perform equal to oligoclonal bands in the diagnosis of multiple sclerosis,” Clin. Chem. Lab. Med. (2018); pii:/j/cclm.ahead-of-print/cclm-2018-0400/cclm-2018-0400.xml, https://doi.org/10.1515/cclm-2018-0400.
G. F. Weber, S. Zawaideh, S. Hikita, et al., “Phosphorylation-dependent interaction of osteopontin with its receptors regulates macrophage migration and activation,” J. Leukoc. Biol., 72, 752–761 (2002), https://doi.org/10.1189/jlb.72.4.752.
L. Börnsen, M. Khademi, T. Olsson, et al., “Osteopontin concentrations are increased in cerebrospinal fluid during attacks of multiple sclerosis,” Mult. Scler., 17, No. 1, 32–42 (2010), https://doi.org/10.1177/1352458510382247.
L. Szalardy, D. Zadori, M. Simu, et al., “Evaluating biomarkers of neuronal degeneration and neuroinflammation in CSF of patients with multiple sclerosis–osteopontin as a potential marker of clinical severity,” J. Neurol. Sci., 331, No. 1–2, 38–42 (2013), https://doi.org/10.1016/j.jns.2013.04.024.
S.-R. Wen, G.-J. Liu, R.-N. Feng, et al., “Increased levels of IL-23 and osteopontin in serum and cerebrospinal fluid of multiple sclerosis patients,” J. Neuroimmunol., 244, No. 1–2, 94–96 (2012), https://doi.org/10.1016/j.jneuroim.2011.12.004.
A. Paul, M. Comabella, and R. Gandhi, “Biomarkers in multiple sclerosis,” Cold Spring Harb. Perspect. Med. a029058 (2018), https://doi.org/10.1101/cshperspect.a029058.
R. Egg, M. Reindl, F. Deisenhammer, et al., “Anti-MOG and anti-MBP antibody subclasses in multiple sclerosis,” Mult. Scler., 7, No. 5, 285–289 (2001), https://doi.org/10.1177/135245850100700503.
B. Olsson, C. Malmeström, H. Basun, et al., “Extreme stability of chitotriosidase in cerebrospinal fluid makes it a suitable marker for microglial activation in clinical trials,” J. Alzheimers Dis., 32, No. 2, 273–276 (2012), https://doi.org/10.3233/JAD-2012-120931.
C. Malmeström, M. Axelsson, J. Lycke, et al., “CSF levels of YKL-40 are increased in MS and decrease with immunosuppressive treatment,” J. Neuroimmunol., 269, No. 1–2, 87–89 (2014), https://doi.org/10.1016/j.jneuroim.2014.02.004.
M. P. Stoop, V. Singh, C. Stingl, et al., “Effects of natalizumab treatment on the cerebrospinal fluid proteome of multiple sclerosis patients,” J. Proteome Res., 12, No. 3, 1101–1107 (2013), https://doi.org/10.1021/pr3012107.
J. Ottervald, B. Franzén, K. Nilsson, et al., “Multiple sclerosis: Identification and clinical evaluation of novel CSF biomarkers,” J. Proteomics, 73, No. 6, 1117–1132 (2010), https://doi.org/10.1016/j.jprot.2010.01.004.
V. K. Harris, N. Donelan, Q. J. Yan, et al., “Cerebrospinal fluid fetuin-A is a biomarker of active multiple sclerosis,” Mult. Scler., 19, No. 11, 1462–1472 (2013), https://doi.org/10.1177/1352458513477923.
M. Gunnarsson, C. Malmeström, M. Axelsson, et al., “Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab,” Ann. Neurol., 69, No. 1, 83–89 (2011), https://doi.org/10.1002/ana.22247.
L. Novakova, M. Axelsson, M. Khademi, et al., “Cerebrospinal fluid biomarkers of inflammation and degeneration as measures of fi ngolimod efficacy in multiple sclerosis,” Mult. Scler., 23, No. 1, 62–71 (2017), https://doi.org/10.1177/1352458516639384.
L. Piccio, R. T. Naismith, K. Trinkaus, et al., “Changes in B- and T-lymphocyte and chemokine levels with rituximab treatment in multiple sclerosis,” Arch. Neurol., 67, No. 6, 707–714 (2010), https://doi.org/10.1001/archneurol.2010.99.
A. H. Cross, R. S. Klein, and L. Piccio, “Rituximab combination therapy in relapsing multiple sclerosis,” Ther. Adv. Neurol. Disord., 5, No. 6, 311–319 (2012), https://doi.org/10.1177/1756285612461165.
M. Axelsson, C. Malmeström, M. Gunnarsson, et al., Immunosuppressive therapy reduces axonal damage in progressive multiple sclerosis,” Mult. Scler., 20, No. 1, 43–50 (2014), https://doi.org/10.1177/1352458513490544.
A. Petzold, M. J. Eikelenboom, D. Gveric, et al., “Markers for different glial cell responses in multiple sclerosis: Clinical and pathological correlations,” Brain, 125, 1462–1473 (2002), https://doi.org/10.1093/brain/awf165.
J. L. Kanter, S. Narayana, P. P. Ho, et al., “Functional inflammatory profi les distinguish myelin-reactive T cells from patients with multiple sclerosis,” Nat. Med., 12, 138–143 (2006), https://doi.org/10.1126.scitranslmed.aaa8038.
H. Garren, W. H. Robinson, E. Krasulová, et al., BHT-3009 Study Group, “Phase 2 trial of a DNA vaccine encoding myelin basic protein for multiple sclerosis,” Ann. Neurol., 63, 611–620 (2008), https://doi.org/10.1002/ana.21370.
F. J. Quintana, M. F. Farez, V. Viglietta, et al., “Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis, Proc. Natl. Acad. Sci. USA, 105, No. 48, 18889–18894 (2008), https://doi.org/10.1073/pnas.0806310105.
M. Hecker, B. Fitzner, M. Wendt, et al., “High-density peptide microarray analysis of IgG autoantibody reactivities in serum and cerebrospinal fluid of multiple sclerosis patients,” Mol. Cell. Proteomics, 15, No. 4, 1360–1380 (2016), https://doi.org/10.1074/mcp.M115.051664.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Nevrologii i Psikhiatrii imeni S. S. Korsakova, Vol. 119, No. 7, Iss. 1, pp. 95–102, July, 2019
Rights and permissions
About this article
Cite this article
Shedko, E.D., Tyumentseva, M.A. Molecular Biomarkers in the Cerebrospinal Fluid in Multiple Sclerosis. Neurosci Behav Physi 50, 527–533 (2020). https://doi.org/10.1007/s11055-020-00932-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-020-00932-z