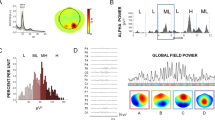
A total of 67 adult subjects took part in studies of the amplitude of the lower (α1) and upper (α2) frequency components of EEG α activity, using individually defined ranges, during execution of circular movements of a computer mouse and during observation of another person carrying out this movement. Topographic specificity was demonstrated for the α2 rhythm, and this rhythm was found to have greater amplitude reactivity than the low-frequency component on execution of movements. The sources of rhythms were identified by low-resolution brain electromagnetic tomography (sLORETA). Both execution and observation of the execution of movements produced similar activation of a number of structures presumptively belonging to the so-called “mirror” system of the brain (precuneus and cingulate gyrus, for the α1 rhythm; inferior parietal lobe, supramarginal and angular gyri, superior and middle temporal gyri, and insular cortex for the α2 rhythm). A significantly greater number of activated voxels was seen in some structures of the right hemisphere than in the left. In the case of executed movements, this phenomenon could be explained in terms of increased needs for subjects’ visuomotor coordination (holding the computer mouse cursor within a specified area of the screen). On observation of movements executed by others, additional activation of the right hemisphere could be due to the social context of the task being performed, supposing attribution of the observed actions to another person. These data can be evaluated as additional evidence supporting the hypothesis of the functional significance of the mirror system of the brain and its involvement in the process of perceiving the movements of another person. The study results also evidence the potential of using a standard computer mouse as an object for operant movement in studies of the neurophysiological mechanisms of motor activity and observations thereof.
Similar content being viewed by others
References
J. A. Pineda, “The functional signifi cance of mu rhythms: Translating ‘seeing’ and ‘hearing’ into ‘doing’,” Brain Res. Rev., 50, No. 1, 57– 68 (2005).
C. Heyes, “Where do mirror neurons come from?” Neurosci. Biobehav. Rev., 34, No. 4, 575–583 (2010).
J. A. Pineda, “Sensorimotor cortex as a critical component of an ‘extended’ mirror neuron system: does it solve the development correspondence and control problems in mirroring?” Behav. Brain Funct., 4, No. 1, 47–63 (2008).
M. Iacoboni, R. P. Woods, M. Brass, et al., “Cortical mechanisms of human imitation,” Science, 286, No. 5449, 2526–2528 (1999).
S. Cochin, C. Barthelemy, S. Roux, and J. Martineau, “Observation and execution of movement: Similarities demonstrated by quantified electroencephalography,” Eur. J. Neurosci., 11, No. 5, 1839–1842 (1999).
S. Frenkel-Toledo, S. Bentin, A. Perry, et al., “Dynamics of the EEG power in the frequency and spatial domains during observation and execution of manual movements,” Brain Res., 1509, 43–57 (2013).
R. Hari and R. Salmelin, “Human cortical oscillations: a neuromagnetic view through the skull,” Trends Neurosci., 20, No. 1, 44–49 (1997).
G. Pfurtscheller, C. Neuper, and G. Krausz, “Functional dissociation of lower and upper frequency mu rhythms in relation to voluntary limb movement,” Clin. Neurophysiol., 111, No. 10, 1873–1879 (2000).
N. E. Crone, D. L. Miglioretti, B. Gordon, et al., “Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. I. Alpha and beta event-related desynchronization,” Brain, 121, No. 12, 2271–2299 (1998).
O. M. Bazanova and D. Vernon, “Interpreting EEG alpha activity,” Neurosci. Biobehav. Rev., 44, 94–110 (2014).
A. Covello, M. de Barros-Ferreira, and G. C. Lairy, “A telemetric study of central rhythms in children,” Electroencephalogr. Clin. Neurophysiol., 38, No. 3, 307–319 (1975).
S. A. Makhin, A. A. Makaricheva, N. V. Lutsuk, and V. B. Pavlenko, “Study of the reactivity of the μ rhythm during observation, auditory perception, and movement imitation: Correlation with empathic ability,” Human Physiol., 41, No. 6, 593–598 (2015).
E. Tognoli and J. A. Kelso, “The coordination dynamics of social neuromarkers,” Front. Hum. Neurosci., 20, No. 9, 563–578 (2015).
D. Posthuma, M. C. Neale, D. I. Boomsma, and E. J. de Geus, “Are smarter brains running faster? Heritability of alpha peak frequency, IQ, and their interrelation,” Behav. Genet., 31, 567–579 (2001).
M. Doppelmayr, W. Klimesch, T. Pachinger, and B. Ripper, “Individual differences in brain dynamics: Important implications for the calculation of event-related band power,” Biol. Cybern., 79, No. 1, 49–57 (1998).
R. Pascual-Marqui, “Standardized low-resolution brain electromagnetic tomography (sLORETA):Technical details,” Methods Find. Exp. Clin. Pharmacol., 24, Suppl. D, 5–12 (2002).
M. Brett, I. S. Johnsrude, and A. M. Owen, “The problem of functional localization in the human brain,” Nat. Rev. Neurosci., 3, No. 3, 243–249 (2002).
J. C. de Munck, S. I. Goncalves, R. Mammoliti, et al., “Interactions between different EEG frequency bands and their effect on alpha–fMRI correlations,” NeuroImage, 47, No. 1, 69–76 (2009).
H. Laufs, A. Kleinschmidt, A. Beyerle, et al., “EEG-correlated fMRI of human alpha activity,” Neuroimage, 19, No. 4, 1463–1476 (2003).
T. E. Nichols and A. P. Holmes, “Nonparametric permutation tests for functional neuroimaging: a primer with examples,” Hum. Brain Mapp., 15, No. 1, 1–25 (2002).
“World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects,” JAMA, 310, No. 20, 2191–2194 (2013).
C. Babiloni, F. Carducci, F. Cincotti, et al., “Human movement-related potentials vs. desynchronization of EEG alpha rhythm: A high-resolution EEG study,” NeuroImage, 10, No. 6, 658–665 (1999).
G. Pfurtscheller, A. Stancák, Jr., and G. Edlinger, “On the existence of different types of central beta rhythms below 30 Hz,” Electroencephalogr. Clin. Neurophysiol., 102, No. 4, 316–325 (1997).
W. Storm van Leeuwen, G. Wieneke, P. Spoelstra, and H. Versteeg, “Lack of bilateral coherence of mu rhythm,” Electroencephalogr. Clin. Neurophysiol., 44, No. 2, 140–146 (1978).
A. Perry and S. Bentin, “Does focusing on hand-grasping intentions modulate electroencephalogram mu and alpha suppressions?” NeuroReport, 21, 1050–1054 (2010).
I. Goldberg, M. Harel, and R. Malach, “When the brain loses its self: prefrontal inactivation during sensorimotor processing,” Neuron, 50, No. 2, 329–39 (2006).
D. S. Margulies, J. L. Vincent, and C. Kelly, “Precuneus shares intrinsic functional architecture in humans and monkeys,” Proc. Natl. Acad. Sci. USA, 106, No. 47, 20,069–20,074 (2009).
T. Paus, “Primate anterior cingulate cortex: Where motor control, drive and cognition interface,” Nat. Rev. Neurosci., 2, No. 6, 417–424 (2001).
R. Mukamel, A. D. Ekstrom, J. Kaplan, et al., “Single-neuron responses in humans during execution and observation of actions,” Curr. Biol., 20, No. 8, 750–756 (2010).
C. Farrer and C. D. Frith, “Experiencing oneself vs another person as being the cause of an action: The neural correlates of the experience of agency,” Neuroimage, 15, No. 3, 596–603 (2002).
G. Silani, C. Lamm, C. C. Ruff, and T. Singer, “Right supramarginal gyrus is crucial to overcome emotional egocentricity bias in social judgments,” J. Neurosci., 33, No. 39, 15,466–15,476 (2013).
M. L. Seghier, “The angular gyrus: Multiple functions and multiple subdivisions,” Neuroscientist, 19, No. 1, 43–61 (2013).
C. Warrier, P. Wong, V. Penhune, et al., “Relating structure to function: Heschl’s gyrus and acoustic processing,” J. Neurosci., 29, No. 1, 61–69 (2009).
J. D. Herrington, C. Nymber, and R. T. Schultz, “Biological motion task performance predicts superior temporal sulcus activity,” Brain Cogn., 77, No. 3, 372–381 (2011).
R. A. Mar, “The neural bases of social cognition and story comprehension,” Annu. Rev. Psychol., 62, 103–134 (2011).
P. Molenberghs, R. Cunnington, and J. B. Mattingley, “Brain regions with mirror properties: A metaanalysis of 125 human fMRI studies,” Neurosci. Biobehav. Rev., 36, No. 1, 341–349 (2012).
K. G. Nadig, L. Jancke, R. Luchinger, and K. Lutz, “Motor and non-motor error and the infl uence of error magnitude on brain activity,” Exp. Brain Res., 202, No. 1, 45–54 (2010).
M. E. J. Campbell, S. Mehrkanoon, and R. Cunnington, “Intentionally not imitating: Insula cortex engaged for top-down control of action mirroring,” Neuropsychologia, 111, 241–251 (2018).
S. Caspers, S. B. Eickhoff, S. Geyer, et al., “The human inferior parietal lobule in stereotaxic space,” Brain Struct. Funct., 212, 481– 495 (2008).
L. Q. Uddin, I. Molnar-Szakacs, E. Zaidel, and M. Iacoboni, “rTMS to the right inferior parietal lobule disrupts self–other discrimination,” Soc. Cogn. Affect. Neurosci., 1, No. 1, 65–71 (2006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 105, No. 3, pp. 311–326, March, 2019.
Rights and permissions
About this article
Cite this article
Nacharova, M.A., Makhin, S.A. & Pavlenko, V.B. Assessment of the Reactivity and Localization of EEG α-Rhythm Frequency Components on Execution and Observation of Movements. Neurosci Behav Physi 50, 468–478 (2020). https://doi.org/10.1007/s11055-020-00922-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-020-00922-1