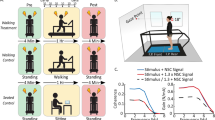
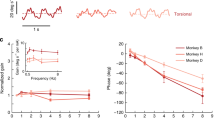
Locomotion is the most important means for movement in space. The role of the vestibular system during human locomotion has received insufficient study, mainly because of difficulties associated with isolated stimulation of this system in these conditions. Isolated stimulation of this system during locomotion is difficult because physical movements of the head, activating the vestibular end organs, unavoidably leads to activation of other sensory inputs. Galvanic stimulation is not a natural means of stimulating the vestibular system, but it has the advantage that it provides isolated stimulation of the vestibular inputs. This technique is relatively new in studies of vestibular involvement in human locomotion. Our review addresses contemporary data on the effects of vestibular signals on locomotion in humans obtained using vestibular galvanic stimulation.
Similar content being viewed by others
References
M. Bute, “Making sense of behavior,” Int. J. Dev. Biol., 42, No. 3, 507–509 (1998).
L. R. Bent, P. S. Bolton, and V. G. Macefield, “Vestibular inputs do not influence the fusimotor system in relaxed muscles of the human leg,” Exp. Brain Res., 180, No. 1, 97–103 (2007).
L. R. Bent, J. T. Inglis, and B. J. McFadyen, “When is vestibular information important during walking?” J. Neurophysiol., 92, No. 3, 1269–1275 (2004).
L. R. Bent, B. J. McFadyen, and J. T. Inglis, “Is the use of vestibular information weighted differently across the initiation of walking?” Exp. Brain Res., 157, No. 4, 407–416 (2004).
L. R. Bent, B. J. McFadyen, and V. F. Merkley, et al., “Magnitude effects of galvanic vestibular stimulation on the trajectory of human gait,” Neurosci. Lett., 279, No. 3, 157–160 (2000).
L. R. Bent, B. J. McFadyen, and J. T. Inglis, “Visual-vestibular interactions in postural control during the execution of a dynamic task,” Exp. Brain Res., 146, No. 4, 490–500 (2002).
J. S. Blouin, C. J. Dakin, K. van den Doel, et al., “Extracting phase-dependent human vestibular reflexes during locomotion using both time and frequency correlation approaches,” J. Appl. Physiol., 111, No. 5, 1484–1490 (2011).
S. B. Bertolami, J. T. Inglis, S. Castellani, et al., “Influence of galvanic vestibular stimulation on postural recovery during sudden falls,” Exp. Brain Res., 205, No. 1, 123–129 (2010).
T. Brandt, “Vestibulopathic gait: you’re better off running than walking,” Curr. Opin. Neurol., 13, No. 1, 3–5 (2000).
T. Brandt, M. Strupp, J. Benson, and M. Dietrich, “Vestibulopathic gait. Walking and running,” Adv. Neurol., 87, 165–172 (2001).
A. N. Carlsen, P. M. Kennedy, K. G. Anderson, et al., “Identifying visual-vestibular contributions during target-directed locomotion,” Neurosci. Lett., 384, No. 3, 217–221 (2005).
A. H. Clarke, “Laboratory testing of the vestibular system,” Curr. Opin. Otolaryngol. Head Neck Surg., 18, No. 5, 425–430 (2010).
B. Cohen, G. P. Martinelli, D. Ogorodnikov, et al., “Sinusoidal galvanic vestibular stimulation (sGVS) induces a vasovagal response in the rat,” Exp. Brain Res., 210, No. 1, 45–55 (2011).
B. Cohen, S. B. Yakushin, and G. R. Holstein, “What does galvanic vestibular stimulation actually activate?” Front. Neurol., No. 3, 148 (2012).
H. S. Cohen, “Vestibular disorders and impaired path integration along a linear trajectory,” J. Vestib. Res., 10, No. 1, 7–15 (2000).
C. J. Dakin, J. T. Inglis, R. Chua, and J. S. Blouin, “Muscle-specific modulation of vestibular refl exes with increased locomotor velocity and cadence,” J. Neurophysiol., 110, No. 1, 86–94 (2013).
C. J. Daki, B. L. Luu, K. van den Doel, et al., “Frequency-specific modulation of vestibular-evoked sway responses in humans,” J. Neurophysiol., 103, No. 2, 1048–1056 (2010).
C. J. Dakin, G. M. Son, J. T. Inglis, and J. S. Blouin, “Frequency response of human vestibular reflexes characterized by stochastic stimuli,” J. Physiol., 583, No. 3, 1117–1127 (2007).
B. L. Day, “Galvanic vestibular stimulation: new uses for an old tool,” J. Physiol., 517, No. 3, 631 (1999).
F. Deriu, E. Tolu, and J. C. Rothwell, “A short latency vestibulomasseteric reflex evoked by electric stimulation over the mastoid in healthy humans,” J. Physiol., 553, No. 1, 267–279 (2003).
N. Deshpande and A. E. Patla, “Postural responses and spatial orientation to neck proprioceptive and vestibular inputs during locomotion in young and older adults,” Exp. Brain Res., 167, No. 3, 468–474 (2005).
N. Deshpande and A. Patla, “Dynamic visual-vestibular integration during goal directed human locomotion,” Exp. Brain Res., 166, No. 2, 237–247 (2005).
N. Deshpande and A. Patla, “Visual-vestibular interaction during goal directed locomotion: effects of aging and blurring vision,” Exp. Brain Res., 176, No. 1, 43–53 (2007).
R. Fitzpatrick, D. Burke, and S. Gandevia, “Loop gain of reflexes controlling human standing measured with the use of postural and vestibular disturbances,” J. Neurophysiol., 76, No. 6, 3994–4008 (1996).
R. C. Fitzpatrick, J. E. Butler, and B. L. Day, “Resolving head rotation for human bipedalism,” Curr. Biol., 16, No. 15, 1509–1514 (2006).
R. C. Fitzpatrick and B. L. Day, “Probing the human vestibular system with galvanic stimulation,” J. Appl. Physiol., 96, No. 6, 2301–2316 (2004).
R. C. Fitzpatrick, D. L. Wardman, and J. L. Taylor, “Effects of galvanic vestibular stimulation during human walking,” J. Physiol., 517, No. 3, 931–939 (1999).
S. Glasauer, M. A. Amorim, E. Vitte, and A. Berthoz, “Goal-directed linear locomotion in normal and labyrinthine-defective subjects,” Exp. Brain Res., 98, No. 2, 323–335 (1994).
J. M. Goldberg, C. E. Smith, and C. Fernandez, “Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey,” J. Neurophysiol., 51, No. 6, 1236–1256 (1984).
J. F. Iles, R. Baderin, R. Tanner, and A. Simon, “Human standing and walking: comparison of the effects of stimulation of the vestibular system,” Exp. Brain Res., 178, No. 2, 151–166 (2006).
K. Jahn, M. S. Strupp, E. Schneider, et al., “Differential effects of vestibular stimulation on walking and running,” Neuroreport, 11, No. 8, 1745–1748 (2000).
P. M. Kennedy, A. N. Carlsen, J. T. Inglis, et al., “Relative contributions of visual and vestibular information on the trajectory of human gait,” Exp. Brain Res., 153, No. 1, 113–117 (2003).
J. Kim and I. S. Curthoys, “Responses of primary vestibular neurons to galvanic vestibular stimulation (GVS) in the anaesthetised guinea pig,” Brain Res. Bull., 64, No. 3, 265–271 (2004).
L. D. Latt, P. J. Sparto, J. M. Furman, and M. S. Redfern, “The steady-state postural response to continuous sinusoidal galvanic vestibular stimulation,” Gait Posture, 18, No. 2, 64–72 (2003).
O. Lowenstein, “The effect of galvanic polarization on the impulsedischarge from sense endings in the isolated labyrinth of the thornback ray (Raja clavata),” J. Physiol., 127, No. 1, 104–117 (1955).
B. J. McFadyen, L. Bouyer, L. R. Bent, and J. T. Inglis, “Visualvestibular influences on loco-motor adjustments for stepping over an obstacle,” Exp. Brain Res., 179, No. 2, 235–243 (2007).
T. Pozzo, A. Berthoz, L. Lefort, and E. Vitte, “Head stabilization during various locomotor tasks in humans. II. Patients with bilateral vestibular deficits,” Exp. Brain Res., 85, No. 1, 208–217 (1991).
A. J. Spence, G. Nicholson-Thomas, and R. Lampe, “Closing the loop in legged neuromechanics: an open-source computer vision controlled treadmill,” J. Neurosci. Meth., 215, No. 2, 164–169 (2013).
R. J. St. George, B. L. Day, and R. C. Fitzpatrick, “Adaptation of vestibular signals for self-motion perception,” J. Physiol., 589, No. 4, 843–853 (2011).
R. J. St. George and R. C. Fitzpatrick, “The sense of self-motion, orientation and balance explored by vestibular stimulation,” J. Physiol., 589, No. 4, 807–813 (2011).
T. Stephan, A. Deutschländer, A. Nolte, et al., “Functional MRI of galvanic vestibular stimulation with alternating currents at different frequencies,” Neuroimage, 26, No. 3, 721–732 (2005).
K. S. Utz, V. Dimova, K. Oppenländer, and G. Kerkhoff, “Electrified minds: transcranial direct current stimulation (tDCS) and galvanic vestibular stimulation (GVS) as methods of non-invasive brain stimulation in neuropsychology: a review of current data and future implications,” Neuropsychol., 48, No. 10, 2789–2810 (2010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 100, No. 6, pp. 684–698, June, 2014.
Rights and permissions
About this article
Cite this article
Stolbkov, Y.K., Gerasimenko, Y.P. Vestibular Influences on Locomotion in Humans: Results of the Use of Transmastoid Galvanic Stimulation. Neurosci Behav Physi 46, 42–50 (2016). https://doi.org/10.1007/s11055-015-0196-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-015-0196-3