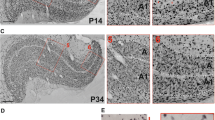
The development of the clustered organization of corticocortical connections between visual field 17 and the posteromedial wall of the lateral suprasylvian sulcus (field PMLS) was studied in cats. The retrograde axonal marker horseradish peroxidase was microinjected into the PMLS region. The distribution of labeled initial neurons was analyzed in field 17 in kittens aged five and 12 weeks. A significant increase in the area of cortex containing labeled neurons and a decrease in their distribution density were seen between week 5 and week 12. Analysis of Fourier amplitude and phase spectra demonstrated differences in the distributions of labeled neurons in kittens of different ages and provided evidence of the incomplete formation of the clustered organization of connections in these animals. The temporal morphofunctional characteristics of the development of intercortical connections of the PMLS zone as compared with other visual areas of the cortex are discussed.
Similar content being viewed by others
References
F. N. Makarov, V. A. Lyakhovetskii, and L. A. Markova, “Ordered structural organization of intrahemisphere connections in the cat visual cortex,” Morfologiya, 124, No. 4, 24–28 (2003).
S. N. Merkulieva and F. N. Makarov, “Ontogenetic characteristics of the organization of corticocortical connections in the primary visual cortex and lateral suprasylvian area of the brain in cats,” Ros. Fiziol. Zh. im. I. M. Sechenova, 96, No. 3, 271–279 (2010).
N. S. Merkulieva and N. I. Nikitina, “Method for detecting and quantifying two-dimensional patterns of the distribution of labeled neurons in the cerebral cortex,” Morfologiya, 138, No. 5, 55–58 (2010).
J. D. Boyd and J. A. Matsubara, “Projections from V1 to lateral suprasylvian cortex: an efferent pathway in the cat’s visual cortex that originates preferentially from CO blob columns,” Vis. Neurosci., 16, 849–860 (1999).
E. M. Callaway and L. C. Katz, “Effects of binocular deprivation on the development of clustered horizontal connections in cat striate cortex,” Proc. Natl. Acad. Sci. USA, 88, 745–749 (1991).
B. Conway, J. D. Boyd, T. H. Stewart, and J. Matsubara, “The projection from V1 to extrastriate area 21a: a second patchy efferent pathway that colocalizes with CO blob columns in cat visual cortex,” Cereb. Cortex, 10, 149–159 (2000).
N. W. Daw, Visual Development, Springer, New York (2006).
B. M. Hooks and C. Chen, “Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse,” Neuron, 52, 281–291 (2006).
G. M. Innocenti and R. Caminiti, “Postnatal shaping of callosal connections from sensory areas,” Exp. Brain Res., 38, 381–394 (1980).
G. M. Innocenti and D. J. Price, “Exuberance in the development of cortical networks,” Nat. Rev. Neurosci., 6, 955–965 (2005).
M.-M. Mesulam, “Tetramethylbenzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents,” J. Histochem. Cytochem., 26, 106–117 (1978).
V. B. Mountcastle, “Modality and topographic properties of single neurons of cat’s somatic sensory cortex,” J. Neurophysiol., 20, 408–434 (1957).
J. Szentagothai, “The module-concept of cerebral cortex architecture,” Brain Res., 95, 475–496 (1975).
J. T. Trachtenberg and M. P. Stryker, “Rapid anatomical plasticity of horizontal connections in the developing visual cortex,” J. Neurosci., 21, No. 10, 3476–3482 (2001).
J. R. Villablanca and C. E. Olmstead, “Neurological development of kittens,” Dev. Psychobiol., 12, No. 2, 101–127 (1979).
T. N. Wiesel and D. H. Hubel, “Effects of visual deprivation on morphology and physiology of cells in the cat’s lateral geniculate body,” J. Neurosci., 26, No. 6, 978–993 (1963).
T. J. Zumbroich and C. Blakemore, “Spatial and temporal selectivity in the suprasylvian visual cortex of the cat,” J. Neurosci., 7, 482–500 (1987).
T. J. Zumbroich, D. J. Price, and C. Blakemore, “Development of spatial and temporal selectivity in the suprasylvian visual cortex of the cat,” J. Neurosci., 8, 2713–2728 (1988).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Morfologiya, Vol. 138, No. 6, pp. 20–23, November–December, 2010.
Rights and permissions
About this article
Cite this article
Merkulieva, N.S., Makarov, F.N. Development of the Ordered Organization of Corticocortical Connections between Field 17 and the Posteromedial Wall of the Lateral Suprasylvian Sulcus in Early Postnatal Ontogeny in Cats. Neurosci Behav Physi 42, 63–66 (2012). https://doi.org/10.1007/s11055-011-9534-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-011-9534-2