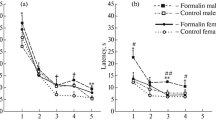
Studies in juvenile (Wistar) rats born to adrenalectomized dams (surgery performed 3–4 weeks before mating) addressed the intensity of behavioral pain responses (numbers of flexion + shaking patterns and durations of licking patterns) induced by foci of inflammation in the formalin test and plasma corticosterone levels on the background of the pain response. Maternal adrenalectomy had no effect on basal corticosterone levels or measures of the intensity of the pain response. In conditions of persistent pain (25 min after injection of formalin), corticosterone levels significantly (p < 0.05) increased in the offspring of both intact and operated dams. Females born to adrenalectomized dams, as compared with females from shamoperated dams, showed higher (p = 0.008) hormone levels in response to persistent pain. There were no differences in measures of the pain responses of the female offspring of adrenalectomized and sham-operated dams. There were no gender differences in measures of the pain response and hormone levels. Thus, pain evoked by acute foci of inflammation in the formalin test activated the hypothalamo-hypophysealadrenal system in 25-day-old rats, though the corticosterone released did not decrease pain intensity, which is consistent with data obtained from the offspring of adrenalectomized dams.
Similar content being viewed by others
References
Yu. G. Balashov, “A fluorimetric micromethod for corticosteroid assay: comparison with other methods,” Fiziol. Zh. SSSR, 76, No. 2, 280–283 (1990).
I. P. Butkevich, V. A. Mikhailenko, and E. A. Vershinina, “Effects of prenatal stress on measures of the behavioral response elicited by a focus of tonic pain in female and male rats during postnatal ontogenesis,” Ros. Fiziol. Zh. im. I. M. Sechenova, 89, No. 9, 1127–1136 (2003).
M. I. Kandror, “Effects of removal of the adrenals in pregnant rats on the functional state of the hypophyseal-adrenal system in their offspring during the first days of postnatal life,” Byull. Éksperim. Biol. Med., 67, No. 3, 37–40 (1969).
L. N. Maslova, “The state of the hypophyseal-adrenal system in rats born to adrenalectomized mothers,” Izv. Sib. Otdel Akad. Nauk SSSR, Sekts. Biol. Nauki, No. 3, Part 3, 130–135 (1983).
G. Sel'e, The Characteristics of Adaptation Syndrome [in Russian], Medgiz, Moscow (1960).
I. P. Butkevich and E. A. Vershinina, “Prenatal stress alters time characteristics and intensity of formalin-induced pain responses in juvenile rats,” Brain Res., 915, No. 1, 88–93 (2001).
T. J. Coderre, J. Katz, A. L. Vaccarino, and R. Melzack, “Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence,” J. Pain, 52, No. 3, 259–285 (1993).
V. Duric and K. E. McCarson, “Persistent pain produces stress-like alterations in hippocampal neurogenesis and gene expression,” J. Pain, 7, No. 8, 544–555 (2006).
G. F. Gebhart, “Descending modulation of pain,” Neurosci. Biobehav. Res., 27, 729–737 (2004).
N. J. van Praag, “Can stress cause depression?” World J. Biol. Psychiatry, 6, Supplement 2, 5–22 (2005).
B. K. Taylor, S. F. Akana, M. A. Peterson, M. F. Dallman, and A. I. Basbaum, “Pituitary-adrenocortical responses to persistent noxious stimuli in the awake rat: endogenous corticosterone does not reduce nociception in the formalin test,” Endocrinology, 139, No. 5, 2407–2413 (1998).
A. Tjølsen, O.-G. Berge, S. Hunskaar, J. H. Rosland, and K. Hole, “The formalin test: an evaluation of the method,” J. Pain, 51, No. 1, 5–17 (1992).
L. S. Williams, W. J. Jones, J. Shen, R. L. Robinson, M. Weinberger, and K. Kroenke, “Prevalence and impact of depression and pain in neurology outpatients,” J. Neurol. Neurosurg. Psychiatry, 74, No. 11, 1587–1589 (2003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 94, No. 3, pp. 312–317, March, 2008.
Rights and permissions
About this article
Cite this article
Butkevich, I.P., Mikhailenko, V.A., Bagaeva, T.R. et al. Persistent pain responses in inflammation and corticosterone levels in juvenile rats born to adrenalectomized dams. Neurosci Behav Physi 39, 297–300 (2009). https://doi.org/10.1007/s11055-009-9129-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-009-9129-3