Abstract
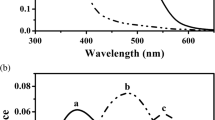
The studies reported here provide the first demonstration that retinal responses in both the fry of the migratory salmon trout Oncorhynchus masou and the dwarf form of this species changed in conditions of experimental neutralization of the geomagnetic field (GMF); migratory salmon trout fry and dwarves showed different changes. The responses of different types of retinal photoreceptor in migratory salmon trout fry to neutralization of the GMF differed: while rods and double cones perceived neutralization of the GMF as the onset of darkness (the scotopic reaction), single (generally blue-sensitive) cones responded to neutralization of the GMF both as presentation of blue light or (very rarely) ultraviolet irradiation. The retina of dwarf male salmon trout responded to neutralization of the GMF with a double response: rods showed a light (photopic) response, while double (red/green-sensitive) cones produced dark (scotopic) responses. Single (blue-sensitive) cones responded to neutralization of the GMF as bright blue light. Thus, the morphological picture of the retina in dwarf male salmon trout in these experimental conditions corresponds to the perception of blue light. The initial conditions were different — normal diffuse daylight with a brightness of about 7.5 Lx. It is likely that neutralization of the magnetic field had no effect on rods, while double, red-green, cones responded as to darkness, i.e., the fish did not perceive red or green light in the visible spectrum, but perceived only blue and, possibly, ultraviolet light by means of central blue-sensitive and accessory cones. Thus, these experiments demonstrated that in conditions of normal daylight illumination, retinal photoreceptors in salmon fry respond to changes in the earth’s magnetic field, i.e., objectively function as magnetoreceptors.
Similar content being viewed by others
References
V. I. Govardovskii and L. V. Zueva, “A simple highly-sensitive recording microspectrophotometer,” Tsitologiya, 30, No. 4, 499–502 (1988).
A. A. Maksimovich, A. A. Kudra, and V. M. Serkov, “Morphological changes in the retina of young salmon trout Oncorhynchus masou in experimental changes in the geomagnetic field,” Tsitologiya, 44, No. 2, 140–150 (2002).
K. Smit, The Biology of the Sensory Systems [in Russian], BINOM, Moscow (2005).
M. A. Ali, “Histophysiological studies on the juvenile Atlantic salmon (Salmo salar) retina. Responses to light intensities, wavelengths, temperatures and continuous light or dark,” Can. J. Zool., 39, 511–526 (1961).
J. Ammermuller, J. F. Miller, and H. Kolb, “The organization of the turtle inner retina. II. Analysis of color-coded and directionally selective cells,” J. Comp. Neurol., 358, No. 1, 35–62 (1995).
L. Beaudet, I. N. Flamarique, and C. W. Hawryshyn, “Cone photoreceptor topography in the retina of sexually mature Pacific salmonid fishes,” J. Comp. Neurol., 383, 49–59 (1997).
J. K. Bowmaker, V. I. Govardovskii, S. A. Shukolyukov, et al., “Visual pigments and the photic environment-the cottoid fish of Lake Baikal,” Vis. Res., 34, 591–605 (1994).
C. Demaine and P. Semm, “The avian pineal gland as an independent magnetic sensor,” Neurosci. Lett., 62, No. 1, 119–122 (1985).
M. E. Deutschlander, D. K. Greaves, T. J. Haimberger, and C. W. Hawryshyn, “Functional mapping of ultraviolet photosensitivity during metamorphic transitions in a salmonid fish, Oncorhynchus mykiss,” J. Exptl. Biol., 204, 2401–2413 (2001).
R. W. Eveson, C. R. Timmel, B. Brocklehurst, et al., “The effect of weak magnetic fields on radical recombination reaction in micelles,” Int. J. Radiat. Biol., 76, No. 11, 1509–1522 (2000).
I. N. Flamarique and C. W. Hawryshyn, “Retinal development and visual sensitivity of young Pacific sockeye salmon (Oncorhynchus nerka),” J. Exptl. Biol., 199, 869–882 (1996).
P. Gouras, “Color Vision,” Prog. Ret. Res., 3, 227–261 (1984).
V. I. Govardovskii, N. Fyhrquist, T. Reuter, et al., “In search of the visual pigment template,” Vis. Neurosci., 17, 509–528 (2000).
H. Kolb and L. Lipetz, “The anatomical basis for colour vision in the vertebrate retina,” in: Vision and Visual Dysfunction. The Perception of Colour, Macmillan Press Ltd., London, Vol. 7, pp. 128–1145 (1991).
K. J. Lohmann and S. Johnsen, “The neurobiology of magnetoreception in vertebrate animals,” Trends Neurosci., 23, 153–159 (2000).
A. Lyall, “Cone arrangement in teleost retina,” Quart. J. Microsc. Sci., 98, 189–201 (1957).
T. Ritz, D. H. Dommer, and J. B. Phillips, “Shedding light on vertebrate magnetoreception,” Neuron, 34, 503–506 (2002).
K. Schulten, C. Swenberg, and A. Weller, “A biomagnetic sensory mechanism based on magnetic field modulated coherent electron spin motion,” Z. Phys. Chem., 111, 1–5 (1978).
P. Semm, D. Nohr, C. Demaine, and W. Wiltschko, “Neural basis of the magnetic compass: interaction of visual, magnetic and vestibular inputs in the pigeon’s brain,” J. Comp. Physiol., A155, 283–288 (1984).
A. J. Sillman, “Avian vision,” in: Avian Biology, Academic Press, New York (1973), Vol. 3, pp. 349–383.
G. Walls, The Vertebrate Eye and Adaptive Radiation, Cranbrooke, Bloomfield Hills, MI (1942).
R. Wiltschko and W. Wiltschko, Magnetic Orientation in Animals, Springer, Berlin (1995).
Author information
Authors and Affiliations
Corresponding author
Additional information
__________
Translated from Morfologiya, Vol. 132, No. 4, pp. 44–51, July–August, 2007.
Rights and permissions
About this article
Cite this article
Maksimovich, A.A., Kondrashev, S.L. & Gnyubkina, V.P. Morphological changes in the retina in Pacific ocean salmon Oncorhynchus masou fry in response to neutralization of the geomagnetic field in conditions of normal illumination. Neurosci Behav Physi 38, 821–827 (2008). https://doi.org/10.1007/s11055-008-9054-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-008-9054-x