Abstract
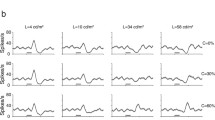
The responses of 51 neurons in the lateral geniculate nucleus of the rabbit to substitution of colored stimuli different brightness and stimuli differing only in intensity were studied. Neurons in the geniculate nucleus, like neurons in the visual cortex, were found to respond with initial phasic discharges at 50–90 msec after stimulus substitution, the magnitudes of these responses correlating with the interstimulus differences; neurons also showed prolonged tonic responses in which the spike frequency depended on the intensity of the stimulus presented. Analysis of phasic responses allowed two groups of neurons to be identified: some were specialized to discriminate stimulus intensity only, while others were specialized to discriminate both the intensity and the color tone of the stimulus. Use of the magnitude of the early phasic discharge as a measure of the difference between stimuli yielded a sensory space for lateral geniculate nucleus neurons. The responses of neurons in the first group (44 cells, 86%) produced a two-dimensional achromatic space with two axes-brightness and darkness; this structure appeared independently of whether stimuli were of the same or different color tones. The phasic responses of neurons in the second group (seven of 51, 14%) generated a four-dimensional space with two color and two achromatic axes. The color and achromatic spaces of lateral geniculate nucleus neurons were analogous to the spaces previously identified for neurons in the rabbit visual cortex using the same stimulation conditions. The sensory spaces reconstructed on the basis of neuron phasic discharges essentially coincided with the spaces obtained from analysis of the N85 component of visual evoked potentials in rabbits, which provides support for the vector information coding principle in the visual analyzer. The tonic discharges of most lateral geniculate nucleus neurons correlated linearly with changes in stimulus intensity and can be regarded as reflecting a pre-detector function for the visual cortex detector neurons.
Similar content being viewed by others
References
A. V. Vartanov, V. B. Polyanskii, E. N. Sokolov, and D. V. Evtikhin, “Characteristics of the color perceptual space of protanomals,” Zh. Vyssh. Nerv. Deyat., 48, No. 5, 788–796 (1998).
A. V. Latanov, A. Yu. Leonova, D. V. Evtikhin, and E. N. Sokolov, “Comparative neurobiology of color vision in humans and animals,” Zh. Vyssh. Nerv. Deyat., 47, No. 2, 308–320 (1997).
V. B. Polyanskii, D. V. Evtikhin, and E. N. Sokolov, “Brightness components of evoked potentials to color stimuli in rabbits,” Zh. Vyssh. Nerv. Deyat., 49, No. 6, 1046–1051 (1999).
V. B. Polyanskii, D. V. Evtikhin, and E. N. Sokolov, “Reconstruction of the brightness and color perceptual space in rabbits on the basis of visual potentials and comparison with data from behavioral experiments,” Zh. Vyssh. Nerv. Deyat., 50, No. 5, 843–854 (2000).
V. B. Polyanskii, D. V. Evtikhin, and E. N. Sokolov, “Calculation of color and brightness differences by human visual cortex neurons,” Zh. Vyssh. Nerv. Deyat., 55, No. 1, 60–70 (2005).
V. B. Polyanskii, G. L. Ruderman, V. V. Gavrilova, E. N. Sokolov, and A. V. Latanov, “Discrimination of light intensity by rabbits and the structure of its achromatic space,” Zh. Vyssh. Nerv. Deyat., 45, No. 5, 957–963 (1995).
V. B. Polyanskii,, E. N. Sokolov, T. Yu. Marchenko, D. V. Evtikhin, and G. L. Ruderman, “The perceptual color space of rabbits,” Zh. Vyssh. Nerv. Deyat., 48, No. 3, 496–504 (1998).
E. N. Sokolov, Perception and Conditioned Reflexes, A New View [in Russian], UMK Psikhologiya, Moscow (2003).
E. N. Sokolov and Ch. A. Izmailov, Color Vision, [in Russian], Moscow State University Press, Moscow (1984).
S. V. Fomin, E. N. Sokolov, and G. G. Vaitkyavichus, Artificial Sensory Organs. Questions in the Modeling of Sensory Systems [in Russian], Nauka, Moscow (1979).
G. H. Jacobs, “The distribution and nature of color vision among the mammals,” Biol. Camb. Philos. Soc., 68, No. 3, 413–471 (1993).
F. H. Jacobs and M. P. Rowe, “Evolution of vertebrate color vision,” Clin. Exptl. Opt., 84, No. 4–5, 206–216 (2004).
J. F. W. Nuboer and P. J. Moed, “Increment-threshold spectral sensitivity in the rabbit,” J. Comp. Physiol., 151, 353–358 (1983).
J. F. W. Nuboer, W. M. van Nuys, and J. F. Wortel, “Cone systems in the rabbit retina revealed by ERG-null detection,” J. Comp. Physiol., 151, 347–352 (1983).
Author information
Authors and Affiliations
Additional information
__________
Translated from Zhurnal Vysshei Nervnoi Deyatel’nosti imeni I. P. Pavlova, Vol. 56, No. 1, pp. 75–85, January–February, 2006.
Rights and permissions
About this article
Cite this article
Polyanskii, V.B., Evtikhin, D.V., Sokolov, E.N. et al. Computation of color and brightness differences by neurons in the lateral geniculate nucleus of the rabbit. Neurosci Behav Physiol 37, 237–247 (2007). https://doi.org/10.1007/s11055-007-0007-6
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11055-007-0007-6