Abstract
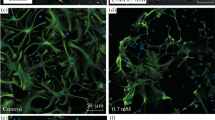
The aim of the present work was to study the location and structural organization of astrocytes in the rat hippocampus, which contain immunoreactive glial fibrillary acid protein (GFAP) after ischemic damage to the brain after intracerebroventricular administration of the neuroprotective agent creatine and without treatment. Light microscopy and immunocytochemical methods were used to study the brains of 26 adult male Sprague-Dawley (Koltushi) rats, some of which were subjected to total cerebral ischemia (12 min) under anesthesia with subsequent reperfusion (seven days). Creatine was given to 11 animals intracerebroventricularly using an osmotic pump (Alzet Osmotic Mini-Pump). The results showed that GFAP-immunoreactive hippocampal astrocytes were concentrated in two main zones (the stratum lacunosummoleculare of field CA1 and the stratum polymorphae of the dentate fascia). The neuroprotective effect of creatine had the result that moderate ischemic damage to the hippocampus did not lead to changes in the zones containing activated astrocytes. The redistribution of GFAP-positive astrocytes in the post-ischemic period was associated with loss of pyramidal neurons in cytoarchitectonic field CA1. Complete loss of pyramidal neurons in this area of the hippocampus leads to a qualitatively new level of astrocyte activation - proliferation.
Similar content being viewed by others
REFERENCES
O. S. Vinogradova, The Hippocampus and Memory [in Russian], Meditsina, Moscow (1975).
D. É. Korzhevskii, “The use of monoclonal antibodies to nuclear protein PCNA for detection of proliferating cells in the developing human brain,” Morfologiya, 118, No.5, 68–70 (2000).
A. P. Novozhilova and O. N. Gaikova, “Cellular gliosis of the white matter of the human brain and its significance in the pathogenesis of focal epilepsy,” Morfologiya, 119, No.2, 20–24 (2001).
A. A. Sosunov, Yu. A. Chelyshev, G. MacKhann, et al., “Neurogenesis in the brains of mature mammals, ” Ontogenez, 33, No.6, 405–420 (2002).
I. Bechmann and R. Nitsch, “Astrocytes and microglial cells incorporate degenerating fibers following entorhinal lesion: a light, confocal, and electron microscopical study using a phagocytosis-dependent labeling technique,” Glia, 20, No.2, 145–154 (1997).
J. A. Colombo, “Interlaminar astroglial processes in the cerebral cortex of adult monkeys but not of adult rats,” Acta Anat., 155, No.1, 57–62 (1996).
E. M. Frohman, S. van der Noort, and S. Gupta, “Astrocytes and intracerebral immune responses” J. Clin. Immunol., 9, No.1, 1–9 (1989).
R. Gadamski and H. Kruoh, “Immunoreactivity of astroglia in the hippocampus of the Mongolian gerbil during short survival following brief ischemia,” Neuropathol. Pol., 30, No.3–4, 221–229 (1992).
P. Gasque, J. Jones, S. K. Singhrao, and B. Morgan, “Identification of an astrocyte cell population from human brain that expresses perforin, a cytotoxic protein implicated in immune defence,” J. Exptl. Med., 187, No.4, 451–460 (1998).
J. L. Ridet, S. K. Malhotra, A. Privat, and F. H. Gage, “Reactive astrocytes: cellular and molecular cues to biological function,” Trends Neurosci., 20, No.12, 570–577 (1997).
D. N. Singh and T. C. Mathew, “Immunocytochemical studies of astrocytes following injury to the cerebral cortex of the rat,” Acta Anat., 134, No.2, 156–159 (1989).
N.-L. Smith, G. Bendek, N. Dahlgren, et al., “Models for studying long-term recovery following forebrain ischemia in the rat. 2. A 2-vessel occlusion model,” Acta Neurol. Scand., 69, No.6, 385–401 (1984).
M. N. Wallace and K. Fredens, “Activated astrocytes of mouse hippocampus contain high levels of NADPH-diaphorase,” Neuroreport, 3, No.11, 953–956 (1992).
L. C. Wei, M. Shi, L. W. Chen, et al., “Nestin-containing cells express glial fibrillary acidic protein in the proliferative regions of central nervous system of postnatal developing and adult mice,” Brain Res. (Dev. Brain Res.), 139, No.1, 9–17 (2002).
Z. C. Ye and H. Sontheimer, “Astrocytes protect neurons from neurotoxic injury by serum glutamate, ” Glia, 22, No.3, 237–248 (1998).
Author information
Authors and Affiliations
Additional information
Translated from Morfologiya, Vol. 125, No. 2, pp. 19–21, March–April, 2004.
Rights and permissions
About this article
Cite this article
Korzhevskii, D.É., Otellin, V.A., Grigor’ev, I.P. et al. Structural organization of astrocytes in the rat hippocampus in the post-ischemic period. Neurosci Behav Physiol 35, 389–392 (2005). https://doi.org/10.1007/s11055-005-0038-9
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11055-005-0038-9