Abstract
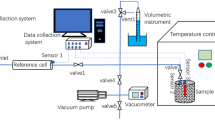
The adsorption kinetic characteristics of CH4, CO2, and N2 in coal provide crucial information for increasing coal seam gas recovery. In this study, isothermal adsorption kinetics experiments were conducted using automatic high-pressure gas adsorption analyzer to reveal differences in adsorption kinetic characteristics of CH4, CO2, and N2 in coal. The research shows that, before reaching adsorption equilibrium, the adsorption ratio of CO2 was higher than that of N2 while that of CH4 was the smallest. The initial adsorption rates of CO2, CH4, and N2 were 1998, 205, and 76 cm3 g−1 h−1, respectively. The order of decreasing gas adsorption rate with respect to the fluctuation zone was CO2 → N2 → CH4. Before reaching adsorption equilibrium, the gas adsorption ratio at high temperature was higher than that at low temperature. The adsorption rates (Rt) of gas were divided into a rapid decay zone (Rt > 0.1 Qe/h), slow decay zone (0.01 Qe/h < Rt < 0.1 Qe/h), and an adsorption equilibrium zone (|Rt| < 0.01 Qe/h). Among the pseudo-first-order kinetic equation, the Fickian diffusion model and the dynamic diffusion model, the latter can best describe the adsorption kinetic process of coal. This work is expected to improve the theoretical basis of enhancing coal seam gas recovery and enrich the theory of gas adsorption in coal.











Similar content being viewed by others
References
Aksu, Z. (2001). Biosorption of reactive dyes by dried activated sludge: equilibrium and kinetic modelling. Biochemical Engineering Journal, 7(1), 79–84.
Chaback, J. J., Morgan, W. D., & Yee, D. (1996). Sorption of nitrogen, methane, carbon dioxide and their mixtures on bituminous coals at in-situ conditions. Fluid Phase Equilibria, 117(1), 289–296.
Cui, G., Tan, Y., Chen, T., Feng, X. T., Elsworth, D., Pan, Z., & Wang, C. (2020). Multidomain two-phase flow model to study the impacts of hydraulic fracturing on shale gas production. Energy & Fuels, 34(4), 4273–4288.
Cui, X., Bustin, R. M., & Dipple, G. M. J. F. (2004). Selective transport of CO2, CH4, and N2 in coals: insights from modeling of experimental gas adsorption data. Fuel, 83(3), 293–303.
Dang, Y., Zhao, L., Lu, X., Xu, J., Sang, P., Guo, S., et al. (2017). Molecular simulation of CO2/CH4 adsorption in brown coal: Effect of oxygen-, nitrogen-, and sulfur-containing functional groups. Applied Surface Science, 423, 33–42.
Day, S., Fry, R., & Sakurovs, R. (2008). Swelling of Australian coals in supercritical CO2. International Journal of Coal Geology, 74(1), 41–52.
Dong, K., Zeng, F., Jia, J., Chen, C., & Gong, Z. (2019). Molecular simulation of the preferential adsorption of CH4 and CO2 in middle-rank coal. Molecular Simulation, 45(1), 15–25.
Fang, H., Sang, S., & Liu, S. (2019). Establishment of dynamic permeability model of coal reservoir and its numerical simulation during the CO2-ECBM process. Journal of Petroleum Science and Engineering, 179, 885–898.
Ghalandari, V., Hashemipour, H., & Bagheri, H. (2020). Experimental and modeling investigation of adsorption equilibrium of CH4, CO2, and N2 on activated carbon and prediction of multi-component adsorption equilibrium. Fluid Phase Equilibria, 508, 112433.
Groszek, A. J. (1997). Heats of adsorption and desorption of CO2, CH4, SO2, O2 and N2 on microporous carbons. Carbon, 35(9), 1399–1450.
Gruszkiewicz, M. S., Naney, M. T., Blencoe, J. G., Cole, D. R., Pashin, J. C., & Carroll, R. E. (2009). Adsorption kinetics of CO2, CH4, and their equimolar mixture on coal from the Black Warrior Basin, West-Central Alabama. International Journal of Coal Geology, 77(1), 23–33.
Han, F., Busch, A., Krooss, B. M., Liu, Z., & Yang, J. (2013). CH4 and CO2 sorption isotherms and kinetics for different size fractions of two coals. Fuel, 108, 137–142.
Ho, Y., & McKay, G. (1998). The kinetics of sorption of basic dyes from aqueous solution by sphagnum moss peat. Canadian Journal of Chemical Engineering, 76(4), 822–827.
Irfan, M. F., Usman, M. R., & Kusakabe, K. (2011). Coal gasification in CO2 atmosphere and its kinetics since 1948: A brief review. Energy, 36(1), 12–40.
Kim, H. J., Shi, Y., He, J., Lee, H.-H., & Lee, C.-H. (2011). Adsorption characteristics of CO2 and CH4 on dry and wet coal from subcritical to supercritical conditions. Chemical Engineering Journal, 171(1), 45–53.
Krooss, B. M., van Bergen, F., Gensterblum, Y., Siemons, N., Pagnier, H. J. M., & David, P. (2002). High-pressure methane and carbon dioxide adsorption on dry and moisture-equilibrated Pennsylvanian coals. International Journal of Coal Geology, 51(2), 69–92.
Larsen, J. W. (2004). The effects of dissolved CO2 on coal structure and properties. International Journal of Coal Geology, 57(1), 63–70.
Li, Z., Liu, D., Cai, Y., & Shi, Y. (2016). Investigation of methane diffusion in low-rank coals by a multiporous diffusion model. Journal of Natural Gas Science and Engineering, 33, 97–107.
Li, Z., Wang, D., & Song, D. (2015). Influence of temperature on dynamic diffusion coefficient of CH4 into coal particles by new diffusion model. Journal of China Coal Society, 40(5), 1055–1064. ((in Chinese)).
Liu, X., Xu, H., Qiu, N., Yang, X., Tian, Z., Li, M., & Xue, Y. (2016). Molecular simulation of CH4, CO2, H2O and N2 molecules adsorption on heterogeneous surface models of coal. Applied Surface Science, 389, 894–905.
Lu, Y., Yang, Z., Li, X., Han, J., & Ji, G. (2015). Problems and methods for optimization of hydraulic fracturing of deep coal beds in China. Chemistry and Technology of Fuels and Oils, 51(1), 41–48.
Macdonald, D. D. (1977). The mathematics of diffusion. In Transient techniques in electrochemistry (pp. 47–67).
Majewska, Z., Ceglarska-Stefańska, G., Majewski, S., & Ziętek, J. (2009). Binary gas sorption/desorption experiments on a bituminous coal: Simultaneous measurements on sorption kinetics, volumetric strain and acoustic emission. International Journal of Coal Geology, 77(1), 90–102.
Mukherjee, M., & Misra, S. (2018). A review of experimental research on Enhanced Coal Bed Methane (ECBM) recovery via CO2 sequestration. Earth-Science Reviews, 179, 392–410.
Nodzeński, A. (1998). Sorption and desorption of gases (CH4, CO2) on hard coal and active carbon at elevated pressures. Fuel, 77(11), 1243–1246.
Nie, B., He, X., & Wang, E. (2000). Diffusion mode of methane gas in coal pores. Mining Safety and Environmental Protection, 27(5), 14–16. ((in Chinese)).
Ozdemir, E., Morsi, B. I., & Schroeder, K. (2003). Importance of volume effects to adsorption isotherms of carbon dioxide on coals. Langmuir, 19(23), 9764–9773.
Ozdemir, E., Morsi, B. I., & Schroeder, K. (2004). CO2 adsorption capacity of argonne premium coals. Fuel, 83(7–8), 1085–1094.
Pini, R., Ottiger, S., Storti, G., & Mazzotti, M. (2009). Pure and competitive adsorption of CO2, CH4 and N2 on coal for ECBM. Energy Procedia, 2009(1), 1705–1710.
Pan, Z., Connell, L. D., Camilleri, M., & Connelly, L. (2010). Effects of matrix moisture on gas diffusion and flow in coal. Fuel, 89(11), 3207–3217.
Rodrigues, C. F. A., Dinis, M. A. P., & Lemos de Sousa, M. J. (2015). Review of European energy policies regarding the recent “carbon capture, utilization and storage” technologies scenario and the role of coal seams. Environmental Earth Sciences, 74(3), 2553–2561.
Ruckenstein, E., Vaidyanathan, A. S., & Youngquist, G. R. (1971). Sorption by solids with bidisperse pore structures. Chemical Engineering Science, 26(9), 1305–1318.
Schoen, M. (1999). The Joule-Thomson effect in confined fluids. Physica A: Statistical Mechanics and its Applications, 270(3), 353–379.
Siemons, N., & Busch, A. (2007). Measurement and interpretation of supercritical CO2 sorption on various coals. International Journal of Coal Geology, 69(4), 229–242.
Sircar, S. (1999). Gibbsian surface excess for gas adsorption revisited. Industrial & Engineering Chemistry Research, 38(10), 3670–3682.
Tang, X., Li, Z., Ripepi, N., Louk, A. K., Wang, Z., & Song, D. (2015). Temperature-dependent diffusion process of methane through dry crushed coal. Journal of Natural Gas Science & Engineering, 22(2015), 609–617.
Wang, L., Wang, Z., Li, K., & Chen, H. (2015). Comparison of enhanced coalbed methane recovery by pure N2 and CO2 injection: Experimental observations and numerical simulation. Journal of Natural Gas Science and Engineering, 23, 363–372.
Wang, Q., Li, W., Zhang, D., Wang, H., Jiang, W., Zhu, L., et al. (2016). Influence of high-pressure CO2 exposure on adsorption kinetics of methane and CO2 on coals. Journal of Natural Gas Science and Engineering, 34, 811–822.
Yu, S., Bo, J., & Fengjuan, L. (2019). Competitive adsorption of CO2/N2/CH4 onto coal vitrinite macromolecular: Effects of electrostatic interactions and oxygen functionalities. Fuel, 235, 23–38.
Yuan, L. (2016). Control of coal and gas outbursts in Huainan mines in China: A review. Journal of Rock Mechanics and Geotechnical Engineering, 8(4), 559–567.
Yuan, L. (2011). Theories and techniques of coal bed methane control in China. Journal of Rock Mechanics and Geotechnical Engineering, 3(4), 343–351.
Yun, J., Xu, F., Liu, L., Zhong, N., & Wu, X. (2012). New progress and future prospects of CBM exploration and development in China. International Journal of Mining Science and Technology, 22(3), 363–369.
Zhao, J., Xu, H., Tang, D., Mathews, J. P., Li, S., & Tao, S. (2016). A comparative evaluation of coal specific surface area by CO2 and N2 adsorption and its influence on CH4 adsorption capacity at different pore sizes. Fuel, 183, 420–431.
Zhang, D., Cui, Y., Li, S., Song, W., & Lin, W. (2011). Adsorption and diffusion behaviors of methane and carbon dioxide on various rank coals. Journal of China Coal Society, 36(10), 1693–1698. ((in Chinese)).
Zhang, Y., Chi, Y., Xing, W., Liu, S., & Song, Y. (2017). Competitive adsorption/desorption of CH4/CO2/N2 mixture on anthracite from China for ECBM operation. Energy Procedia, 105, 4289–4294.
Acknowledgments
This research was supported financially by The National Natural Science Foundation of China (Grant Nos. 5173-4007, 5167-4192, and 5187-4236) and The National Natural Science Foundation of Shaanxi Province (Grant Nos. 2020JC-48, 2019JLP-02).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sun, W., Lin, H., Li, S. et al. Experimental Research on Adsorption Kinetic Characteristics of CH4, CO2, and N2 in Coal from Junggar Basin, China, at Different Temperatures. Nat Resour Res 30, 2255–2271 (2021). https://doi.org/10.1007/s11053-021-09812-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11053-021-09812-w