Abstract
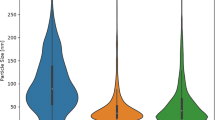
The increasing use of nanopesticides has raised concerns about their effects on crop plants and the impact of human health as well as ecological effects. While increased uptake of metal ions has been observed before, to date, very few studies have demonstrated the presence of nanoparticles in edible tissues. Single-particle inductively coupled plasma–mass spectrometry (sp-ICP-MS) has been suggested as a powerful tool to detect inorganic nanoparticles (NPs) in environmental samples. Here, we exposed edible plant tissues from lettuce, kale, and collard green to nano-CuO, simulating its use as a nanopesticide. We applied sp-ICP-MS to demonstrate the presence of nanoparticles, both in the water used to rinse crop leaf surfaces exposed to nano-CuO and within the leaf tissues. Lettuces retained the highest amounts of nCuO NPs on the leaf surface, followed by collard green and then kale. Surface hydrophilicity and roughness of the leaf surfaces played an important role in retaining nano-CuO. The results indicate that most of the nanoparticles are removed via washing, but that a certain fraction is taken up by the leaves and can result in human exposure, albeit at low levels.

ᅟ









Similar content being viewed by others
References
Adeleye AS, Conway JR, Perez T, Rutten P, Keller AA (2014) Influence of extracellular polymeric substances on the long-term fate, dissolution, and speciation of copper-based nanoparticles. Environ Sci Technol 48:12561–12568
Adeleye AS, Oranu EA, Tao MY, Keller AA (2016) Release and detection of nanosized copper from a commercial antifouling paint. Water Res 102:374–382. https://doi.org/10.1016/j.watres.2016.06.056
Azodi M, Sultan Y, Ghoshal S (2016) Dissolution behavior of silver nanoparticles and formation of secondary silver nanoparticles in municipal wastewater by single particle ICP-MS. Environ Sci Technol 50:13318–13327. https://doi.org/10.1021/acs.est.6b03957
Bornhoft NA, Sun TY, Hilty LM, Nowack B (2016) A dynamic probabilistic material flow modeling method. Environ Model Softw 76:69–80. https://doi.org/10.1016/j.envsoft.2015.11.012
Burns JL, Yan YD, Jameson GJ, Biggs S (1997) A light scattering study of the fractal aggregation behavior of a model colloidal system. Langmuir 13:6413–6420. https://doi.org/10.1021/la970303f
Chen KL, Elimelech M (2006) Aggregation and deposition kinetics of fullerene (C60) nanoparticles. Langmuir 22:10994–11001
Chowdhury I, Cwiertny DM, Walker SL (2012) Combined factors influencing the aggregation and deposition of nano-TiO2 in the presence of humic acid and Bacteria. Environ Sci Technol 46:6968–6976. https://doi.org/10.1021/es2034747
Conway JR, Beaulieu AL, Beaulieu NL, Mazer SJ, Keller AA (2015a) Environmental stresses increase photosynthetic disruption by metal oxide nanomaterials in a soil-grown plant. ACS Nano 9:11737–11749. https://doi.org/10.1021/acsnano.5b03091
Conway JR, Adeleye AS, Gardea-Torresdey J, Keller AA (2015b) Aggregation, dissolution, and transformation of copper nanoparticles in natural waters. Environ Sci Technol 49:2749–2756. https://doi.org/10.1021/es504918q
Dale AL, Lowry GV, Casman EA (2015) Stream dynamics and chemical transformations control the environmental fate of silver and zinc oxide nanoparticles in a watershed-scale model. Environ Sci Technol 49(12):7285–7293 American Chemical Society. https://doi.org/10.1021/acs.est.5b01205
Dan YB, Zhang WL, Xue RM, Ma XM, Stephan C, Shi HL (2015) Characterization of gold nanoparticle uptake by tomato plants using enzymatic extraction followed by single-particle inductively coupled plasma-mass spectrometry analysis. Environ Sci Technol 49:3007–3014. https://doi.org/10.1021/es506179e
Domingos RF, Tufenkji N, Wilkinson KJ (2009) Aggregation of titanium dioxide nanoparticles: role of a fulvic acid. Environ Sci Technol 43:1282–1286. https://doi.org/10.1021/es8023594
El-Habbaa G, Abdou M, El-Shaery S (2016) Biological and chemical control of grapevine die-back disease and their effect on defense related enzymes. Int J Sci Eng Res 7:345–351
Elmer WH, White JC (2016) The use of metallic oxide nanoparticles to enhance growth of tomatoes and eggplants in disease infested soil or soilless medium. Environ Sci: Nano 3:1072–1079
Frechette-Viens L, Hadioui M, Wilkinson KJ (2017) Practical limitations of single particle ICP-MS in the determination of nanoparticle size distributions and dissolution: case of rare earth oxides. Talanta 163:121–126. https://doi.org/10.1016/j.talanta.2016.10.093
Furmidge C (1962) Physico-chemical studies on agricultural sprays. IV.—the retention of spray liquids on leaf surfaces. J Sci Food Agric 13:127–140
Gallego-Urrea JA, Tuoriniemi J, Hassellov M (2011) Applications of particle-tracking analysis to the determination of size distributions and concentrations of nanoparticles in environmental, biological and food samples. Trac-Trend Anal Chem 30:473–483. https://doi.org/10.1016/j.trac.2011.01.005
Gardea-Torresdey JL, Rico CM, White JC (2014) Trophic transfer, transformation, and impact of engineered nanomaterials in terrestrial environments. Environ Sci Technol 48:2526–2540. https://doi.org/10.1021/es4050665
Garner KL, Suh S, Keller AA (2017) Assessing the risk of engineered nanomaterials in the environment: development and application of the nanoFate model. Environ Sci Technol 51(10):5541–5551. https://doi.org/10.1021/acs.est.6b05279
Gottschalk F, Nowack B (2011) The release of engineered nanomaterials to the environment. J Environ Monit 13:1145–1155. https://doi.org/10.1039/c0em00547a
Gottschalk F, Sun TY, Nowack B (2013) Environmental concentrations of engineered nanomaterials: review of modeling and analytical studies. Environ Pollut 181:287–300. https://doi.org/10.1016/j.envpol.2013.06.003
Hadioui M, Merdzan V, Wilkinson KJ (2015) Detection and characterization of ZnO nanoparticles in surface and waste waters using single particle ICPMS. Environ Sci Technol 49:6141–6148. https://doi.org/10.1021/acs.est.5b00681
Hassellöv M, Kaegi R (2009) Analysis and Characterization of Manufactured Nanoparticles in Aquatic Environments. In: Analysis and characterization of manufactured nanoparticles in aquatic environments. Wiley, Hoboken
Hernandez-Viezcas JA, Castillo-Michel H, Peralta-Videa JR, Gardea-Torresdey JL (2016) Interactions between CeO2 nanoparticles and the desert plant mesquite: a spectroscopy approach. ACS Sustain Chem Eng 4:1187–1192
Huang YX, Zhao LJ, Keller AA (2017) Interactions, transformations, and bioavailability of Nano-copper exposed to root exudates. Environ Sci Technol 51:9774–9783. https://doi.org/10.1021/acs.est.7b02523
Keller AA, Lazareva A (2014) Predicted releases of engineered nanomaterials: from global to regional to local. Environ Sci Technol Lett 1:65–70. https://doi.org/10.1021/ez400106t
Keller AA, Wang H, Zhou D, Lenihan HS, Cherr G, Cardinale BJ, Miller R, Ji Z (2010) Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ Sci Technol 44:1962–1967. https://doi.org/10.1021/es902987d
Keller AA, McFerran S, Lazareva A, Suh S (2013) Global life cycle releases of engineered nanomaterials. J Nanopart Res 15:1692
Keller AA, Adeleye AS, Conway JR, Garner KL, Zhao L, Cherr GN, Hong J, Gardea-Torresdey JL, Godwin HA, Hanna S, Ji Z, Kaweeteerawat C, Lin S, Lenihan HS, Miller RJ, Nel AE, Peralta-Videa JR, Walker SL, Taylor AA, Torres-Duarte C, Zink JI, Zuverza-Mena N (2017) Comparative environmental fate and toxicity of copper nanomaterials. Nano 7:28–40. https://doi.org/10.1016/j.impact.2017.05.003
Laborda F, Jimenez-Lamana J, Bolea E, Castillo JR (2013) Critical considerations for the determination of nanoparticle number concentrations, size and number size distributions by single particle ICP-MS. J Anal Atom Spectrom 28:1220–1232. https://doi.org/10.1039/c3ja50100k
Laborda F, Bolea E, Jiménez-Lamana J (2016) Single particle inductively coupled plasma mass spectrometry for the analysis of inorganic engineered nanoparticles in environmental samples. Trends Environ Anal Chem 9:15–23
Larue C, Castillo-Michel H, Sobanska S, Trcera N, Sorieul S, Cécillon L, Ouerdane L, Legros S, Sarret G (2014) Fate of pristine TiO2 nanoparticles and aged paint-containing TiO2 nanoparticles in lettuce crop after foliar exposure. J Hazard Mater 273:17–26. https://doi.org/10.1016/j.jhazmat.2014.03.014
Lazareva A, Keller AA (2014) Estimating potential life cycle releases of engineered nanomaterials from wastewater treatment plants. ACS Sustain Chem Eng 2:1656–1665. https://doi.org/10.1021/sc500121w
Lee S, Bi XY, Reed RB, Ranville JF, Herckes P, Westerhoff P (2014) Nanoparticle size detection limits by single particle ICP-MS for 40 elements. Environ Sci Technol 48:10291–10300. https://doi.org/10.1021/es502422v
Linsinger TPJ, Peters R, Weigel S (2014) International interlaboratory study for sizing and quantification of ag nanoparticles in food simulants by single-particle ICPMS. Anal Bioanal Chem 406:3835–3843. https://doi.org/10.1007/s00216-013-7559-9
Liu HH, Bilal M, Cohen Y, Lazareva A, Keller A (2014) Regional multimedia distribution of nanomaterials and associated exposures: a software platform. In Bioinformatics and Biomedicine (BIBM), 2014 IEEE International Conference on p 10–17
Liu HH, Bilal M, Lazareva A, Keller AA, Cohen Y (2015) Simulation tool for assessing the release and environmental distribution of nanomaterials. Beilstein J Nanotechnol 6(1):938–951 Beilstein-Institut. https://doi.org/10.3762/bjnano.6.97
Liu JY, Murphy KE, Winchester MR, Hackley VA (2017) Overcoming challenges in single particle inductively coupled plasma mass spectrometry measurement of silver nanoparticles. Anal Bioanal Chem 409:6027–6039. https://doi.org/10.1007/s00216-017-0530-4
Ma CX, White JC, Dhankher OP, Xing BS (2015) Metal-based nanotoxicity and detoxification pathways in higher plants. Environ Sci Technol 49:7109–7122. https://doi.org/10.1021/acs.est.5b00685
Meesters JAJ, Koelmans AA, Quik JTK, Hendriks AJ, van de Meent D (2014) Multimedia modeling of engineered nanoparticles with simplebox4nano: model definition and evaluation. Environ Sci Technol 48(10):5726–5736. American Chemical Society. https://doi.org/10.1021/es500548h
Mehrabi K, Nowack B, Dasilya YAR, Mitrano DM (2017) Improvements in nanoparticle tracking analysis to measure particle aggregation and mass distribution: a case study on engineered nanomaterial stability in incineration landfill leachates. Environ Sci Technol 51:5611–5621. https://doi.org/10.1021/acs.est.7b00597
Mitrano DM, Barber A, Bednar A, Westerhoff P, Higgins CP, Ranville JF (2012) Silver nanoparticle characterization using single particle ICP-MS (SP-ICP-MS) and asymmetrical flow field flow fractionation ICP-MS (AF4-ICP-MS). J Anal Atom Spectrom 27:1131–1142. https://doi.org/10.1039/c2ja30021d
Mitrano DM, Ranville JF, Bednar A, Kazor K, Hering AS, Higgins CP (2014) Tracking dissolution of silver nanoparticles at environmentally relevant concentrations in laboratory, natural, and processed waters using single particle ICP-MS (spICP-MS). Environ Sci-Nano 1:248–259. https://doi.org/10.1039/c3en00108c
Mitrano DM, Mehrabi K, Dasilva YAR, Nowack B (2017) Mobility of metallic (nano)particles in leachates from landfills containing waste incineration residues. Environ Sci-Nano 4:480–492. https://doi.org/10.1039/c6en00565a
Montano MD, Olesik JW, Barber AG, Challis K, Ranville JF (2016) Single particle ICP-MS: advances toward routine analysis of nanomaterials. Anal Bioanal Chem 408:5053–5074. https://doi.org/10.1007/s00216-016-9676-8
Nowack B (2017) Evaluation of environmental exposure models for engineered nanomaterials in a regulatory context. Nano 8:38–47. https://doi.org/10.1016/j.impact.2017.06.005
Peters RJB, van Bemmel G, Herrera-Rivera Z, Helsper HPFG, Marvin HJP, Weigel S, Tromp PC, Oomen AG, Rietveld AG, Bouwmeester H (2014) Characterization of titanium dioxide nanoparticles in food products: analytical methods to define nanoparticles. J Agric Food Chem 62:6285–6293. https://doi.org/10.1021/jf5011885
Plathe KL, von der Kammer F, Hassellov M, Moore JN, Murayama M, Hofmann T, Hochella MF (2013) The role of nanominerals and mineral nanoparticles in the transport of toxic trace metals: field-flow fractionation and analytical TEM analyses after nanoparticle isolation and density separation. Geochim Cosmochim Acta 102:213–225. https://doi.org/10.1016/j.gca.2012.10.029
Praetorius A, Scheringer M, Hungerbuhler K (2012) Development of environmental fate models for engineered nanoparticles—a case study of TiO2 nanoparticles in the Rhine River. Environ Sci Technol 46(12):6705–6713. https://doi.org/10.1021/es204530n
Praetorius A, Gundlach-Graham A, Goldberg E, Fabienke W, Navratilova J, Gondikas A, Kaegi R, Günther D, Hofmann T, von der Kammer F (2017) Single-particle multi-element fingerprinting (spMEF) using inductively-coupled plasma time-of-flight mass spectrometry (ICP-TOFMS) to identify engineered nanoparticles against the elevated natural background in soils. Environ Sci-Nano 4:307–314. https://doi.org/10.1039/c6en00455e
Reed RB, Higgins CP, Westerhoff P, Tadjiki S, Ranville JF (2012) Overcoming challenges in analysis of polydisperse metal-containing nanoparticles by single particle inductively coupled plasma mass spectrometry. J Anal Atom Spectrom 27:1093–1100. https://doi.org/10.1039/c2ja30061c
Rui YK, Zhang P, Zhang Y, Ma Y, He X, Gui X, Li Y, Zhang J, Zheng L, Chu S, Guo Z, Chai Z, Zhao Y, Zhang Z (2015) Transformation of ceria nanoparticles in cucumber plants is influenced by phosphate. Environ Pollut 198:8–14. https://doi.org/10.1016/j.envpol.2014.12.017
Salma B, Devi N, Marak T, Nath P, Saha J (2015) In vitro efficacy of some commercial fungicides against Colletotrichum capsici, the causal agent of anthracnose of chilli. Environ Ecol 33:1863–1866
Sannac S (2015) Single particle analysis of nanomaterials using the Agilent 7900 ICP-MS An Agilent Application Note
Schwertfeger DM, Velicogna JR, Jesmer AH, Scroggins RP, Princz JI (2016) Single particle-inductively coupled plasma mass spectroscopy analysis of metallic nanoparticles in environmental samples with large dissolved analyte fractions. Anal Chem 88:9908–9914. https://doi.org/10.1021/acs.analchem.6b02716
Shang J, Gao XH (2014) Nanoparticle counting: towards accurate determination of the molar concentration. Chem Soc Rev 43:7267–7278. https://doi.org/10.1039/c4cs00128a
Song RS, Qin YW, Suh S, Keller AA (2017) Dynamic model for the stocks and release flows of engineered nanomaterials. Environ Sci Technol 51:12424–12433. https://doi.org/10.1021/acs.est.7b01907
Stampoulis D, Sinha SK, White JC (2009) Assay-dependent phytotoxicity of nanoparticles to plants. Environ Sci Technol 43:9473–9479. https://doi.org/10.1021/es901695c
Su YM, Adeleye AS, Keller AA, Huang YX, Dai CM, Zhou XF, Zhang YL (2015) Magnetic sulfide-modified nanoscale zerovalent iron (S-nZVI) for dissolved metal ion removal. Water Res 74:47–57. https://doi.org/10.1016/j.watres.2015.02.004
Sun TY, Gottschalk F, Hungerbuhler K, Nowack B (2014) Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials. Environ Pollut 185:69–76. https://doi.org/10.1016/j.envpol.2013.10.004
Sun TY, Mitrano DM, Bornhoft NA, Scheringer M, Hungerbuhler K, Nowack B (2017) Envisioning Nano release dynamics in a changing world: using dynamic probabilistic modeling to assess future environmental emissions of engineered nanomaterials. Environ Sci Technol 51:2854–2863. https://doi.org/10.1021/acs.est.6b05702
Thio BJR, Zhou DX, Keller AA (2011) Influence of natural organic matter on the aggregation and deposition of titanium dioxide nanoparticles. J Hazard Mater 189:556–563. https://doi.org/10.1016/j.jhazmat.2011.02.072
Tiraferri A, Chen KL, Sethi R, Elimelech M (2008) Reduced aggregation and sedimentation of zero-valent iron nanoparticles in the presence of guar gum. J Colloid Interface Sci 324:71–79. https://doi.org/10.1016/j.jcis.2008.04.064
Trujillo-Reyes J, Majumdar S, Botez C, Peralta-Videa J, Gardea-Torresdey J (2014) Exposure studies of core–shell Fe/Fe 3 O 4 and cu/CuO NPs to lettuce (Lactuca sativa) plants: are they a potential physiological and nutritional hazard? J Hazard Mater 267:255–263
Trumbo P, Yates AA, Schlicker S, Poos M (2001) Dietary reference intakes: vitamin a, vitamin K, arsenic, boron, chromium, copper, iodine, Iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc 101(3):294–301. https://doi.org/10.1016/S0002-8223(01)00078-5.
Zhao L, Huang Y, Hannah-Bick C, Fulton AN, Keller AA (2016a) Application of metabolomics to assess the impact of Cu(OH)2 nanopesticide on the nutritional value of lettuce (Lactuca sativa): enhanced Cu intake and reduced antioxidants. Nanoimpact 3–4:58–66. https://doi.org/10.1016/j.impact.2016.08.005
Zhao L, Ortiz C, Adeleye AS, Hu Q, Zhou H, Huang Y, Keller AA (2016b) Metabolomics to detect response of lettuce (Lactuca sativa) to cu(OH)2 Nano-pesticides: oxidative stress response and detoxification mechanisms. Environ Sci Technol 50:9697–9707. https://doi.org/10.1021/acs.est.6b02763
Zhao L, Huang Y, Zhou H, Adeleye AS, Wang H, Ortiz C, Mazer SJ, Keller AA (2016c) GC-TOF-MS based metabolomics and ICP-MS based metallomics of cucumber (Cucumis sativus) fruits reveal alteration of metabolites profile and biological pathway disruption induced by nano copper. Environ Sci: Nano 3:1114–1123. https://doi.org/10.1039/C6EN00093B
Zhao L, Huang Y, Hu J, Zhou H, Adeleye AS, Keller AA (2016d) 1H NMR and GC-MS based metabolomics reveal defense and detoxification mechanism of cucumber plant under nano-Cu stress. Environ Sci Technol 50:2000–2010. https://doi.org/10.1021/acs.est.5b05011
Zhao L, Hu Q, Huang Y, Fulton A, Hannah-Bick C, Adeleye A, Keller AA (2017a) Activation of antioxidant and detoxification gene expression in cucumber plants exposed to a cu(OH)2 nanopesticide. Environ Sci: Nano 4:1750–1760. https://doi.org/10.1039/C7EN00358G
Zhao L, Hu J, Huang Y, Wang H, Adeleye A, Ortiz C, Keller AA (2017b) 1H NMR and GC–MS based metabolomics reveal nano-Cu altered cucumber (Cucumis sativus) fruit nutritional supply. Plant Physiol Biochem 110:138–146. https://doi.org/10.1016/j.plaphy.2016.02.010
Zhao L, Huang Y, Adeleye AS, Keller AA (2017c) Metabolomics reveals cu(OH)2 Nanopesticide activated antioxidative pathways and decreased beneficial antioxidants in spinach leaves. Environ Sci Technol 51:10184–10194. https://doi.org/10.1021/acs.est.7b02163
Zhao L, Hu Q, Huang Y, Keller AA (2017d) Response at genetic, metabolic, and physiological levels of maize (Zea mays) exposed to a cu(OH)2 Nanopesticide. ACS Sustain Chem Eng 5:8294–8301. https://doi.org/10.1021/acssuschemeng.7b01968
Zhao L, Huang Y, Keller AA (2017e) Comparative metabolic response between cucumber (Cucumis sativus) and corn (Zea mays) to a Cu(OH)2 nanopesticide. J Agr Food Chem. https://doi.org/10.1021/acs.jafc.7b01306
Zhou DX, Ji ZX, Jiang XM, Dunphy DR, Brinker J, Keller AA (2013) Influence of material properties on TiO2 nanoparticle agglomeration. Plos One 8. https://doi.org/10.1371/journal.pone.0081239
Zuverza-Mena N, Medina-Velo IA, Barrios AC, Tan WJ, Peralta-Videa JR, Gardea-Torresdey JL (2015) Copper nanoparticles/compounds impact agronomic and physiological parameters in cilantro (Coriandrum sativum). Environ Sci-Proc Imp 17:1783–1793. https://doi.org/10.1039/c5em00329f
Acknowledgements
Any opinions, findings, conclusions, or recommendations expressed in this material are those of the authors do not necessarily reflect the views of the funding agencies. AAK also appreciates Agilent Technologies for their Agilent Thought Leader Award. The MRL Central Facilities supported by the MRSEC Program of the National Science Foundation under awards NO. DMR 1121053, a member of the NSF-funded Materials Research Facilities Network (www.mrfn.org). We thank the MRL Central Facilities for the use of their TEM instruments and Dr. Aidan Taylor at the UCSB MRL for his help with the TEM measurements.
Funding
This study was funded by National Science Foundation (NSF) and the U.S. Environmental Protection Agency (EPA) under NSF-EF0830117.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
This article is part of the topical collection: 20th Anniversary Issue: From the editors
Nicola Pinna, Executive Editor, Mike Roco, Editor-in-Chief
Electronic supplementary material
ESM 1
(DOCX 716 kb)
Rights and permissions
About this article
Cite this article
Keller, A.A., Huang, Y. & Nelson, J. Detection of nanoparticles in edible plant tissues exposed to nano-copper using single-particle ICP-MS. J Nanopart Res 20, 101 (2018). https://doi.org/10.1007/s11051-018-4192-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-018-4192-8