Abstract
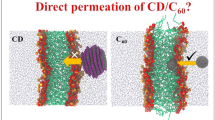
Carbon-based nanoparticles (NPs) such as fullerenes and nanotubes have been extensively studied for drug delivery in recent years. The permeation process of fullerene and its derivative molecules through membrane is essential to the utilization of fullerene-based drug delivery system, but the mechanism and the dynamics of permeation through cell membrane are still unclear. In this study, coarse-grained molecular dynamics simulations were performed to investigate the permeation process of functionalized fullerene molecules (ca. 0.72 nm) through the membrane. Our results show that single functionalized fullerene molecule in such nanoscale could permeate the lipid membrane in micro-second time scale. Pristine C60 molecules prefer to aggregate into several small clusters while C60OH15 molecules could aggregate into one big cluster to permeate through the lipid membrane. After permeation of C60 or its derivatives into membrane, all C60 and C60OH15 molecules disaggregated and monodispersed in the lipid membrane.

ᅟ









Similar content being viewed by others
References
Allen TM, Cullis PR (2004) Drug delivery systems: entering the mainstream. Science 303:1818–1822
Araghi H, Zabihi Z (2013a) Influence of impact angle on the interaction between Co 55 nanocluster and Cu (001) substrate: ionized cluster beam deposition. Comp Mater Sci 67:109–112
Araghi H, Zabihi Z (2013b) Molecular dynamics simulation of microscopic processes in Co nanocluster impact onto Cu (001) substrate. Nucl Instrum Meth B 298:13–18
Bao G, Mitragotri S, Tong S (2013) Multifunctional nanoparticles for drug delivery and molecular imaging. Annu Rev Biomed Eng 15:253–282
Berendsen HJC, Postma JPM, van Gunsteren WF, DiNola A, Haak JR (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81:3684–3690
Bianco A, Kostarelos K, Prato M (2005) Applications of carbon nanotubes in drug delivery. Curr Opin Chem Biol 9:674–679
Bond PJ, Holyoake J, Ivetac A, Khalid S, Sansom MSP (2007) Coarse-grained molecular dynamics simulations of membrane proteins and peptides. J Struct Biol 157:593–605
Brigger I, Dubernet C, Couvreur P (2012) Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliver Rev 64:24–36
Brunetti V, Bouchet LM, Strumia MC (2015) Nanoparticle-cored dendrimers: functional hybrid nanocomposites as a new platform for drug delivery systems. Nanoscale 7:3808–3816
Cha C, Shin SR, Annabi N, Dokmeci MR, Khademhosseini A (2013) Carbon-based nanomaterials: multifunctional materials for biomedical engineering. ACS Nano 7:2891–2897
Chen I, Dubnau D (2004) DNA uptake during bacterial transformation. Nat Rev Microbio 2:241–249
Cheng R, Meng F, Deng C, Klok H-A, Zhong Z (2013) Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 34:3647–3657
DeVane R, Jusufi A, Shinoda W, C-c C, Nielsen SO, Moore PB, Klein ML (2010) Parametrization and application of a coarse grained force field for benzene/fullerene interactions with lipids. J Phys Chem B 114:16364–16372
Dicheva BM, ten Hagen TLM, Seynhaeve ALB, Amin M, Eggermont AMM, Koning GA (2015) Enhanced specificity and drug delivery in tumors by cRGD-anchoring thermosensitive liposomes. Pharm Res 32:3862–3876
Eslami H, Müller-Plathe F (2013) How thick is the interphase in an ultrathin polymer film? Coarse-grained molecular dynamics simulations of polyamide-6, 6 on graphene. J Phys Chem C 117:5249–5257
Eslami H, Jaafari B, Mehdipour N (2013) Coarse grained molecular dynamics simulation of nanoconfined water. Chem Phys Chem 14:1063–1070
Felice B, Prabhakaran MP, Rodríguez AP, Ramakrishna S (2014) Drug delivery vehicles on a nano-engineering perspective. Mater Sci Eng: C 41:178–195
Feng S-S (2014) Nanoparticles of biodegradable polymers for new-concept chemotherapy. Expert Rev Med Devic
Giménez C et al (2015) Gated mesoporous silica nanoparticles for the controlled delivery of drugs in cancer cells. Langmuir 31:3753–3762
Hess B, Kutzner C, Van Der Spoel D, Lindahl E (2008) GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem Theo Comput 4:435–447
Hörmann K, Zimmer A (2016) Drug delivery and drug targeting with parenteral lipid nanoemulsions—a review. J Controll Release 223:85–98
Inui N, Mochiji K, Moritani K, Nakashima N (2010) Molecular dynamics simulations of nanopore processing in a graphene sheet by using gas cluster ion beam. Appl Phys A Mater Sci Process 98:787–794
Jia G et al (2005) Cytotoxicity of carbon nanomaterials: single-wall nanotube, multi-wall nanotube, and fullerene. Environ Sci Technol 39:1378–1383
Johnstone TC, Suntharalingam K, Lippard SJ (2016) The next generation of platinum drugs: targeted Pt (II) agents, nanoparticle delivery, and Pt (IV) prodrugs. Chem Rev 116:3436–3486
Kamaly N, Yameen B, Wu J, Farokhzad OC (2016) Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem Rev 116:2602–2663
Kang JW, Choi KS, Kang JC, Kang ES, Byun KR, Hwang HJ (2001) Cluster deposition study by molecular dynamics simulation: Al and Cu cluster. J Vac Sci Technol A 19:1902–1906
Kotyk A (2012) Cell membrane transport: principles and techniques. Springer Science & Business Media, Berlin
Kreuter J (2014) Drug delivery to the central nervous system by polymeric nanoparticles: what do we know? Adv Drug Deliv Rev 71:2–14
Lee SJ, Jeong Y-I, Park H-K, Kang DH, Oh J-S, Lee S-G, Lee HC (2015) Enzyme-responsive doxorubicin release from dendrimer nanoparticles for anticancer drug delivery. Int J Nanomedicine 10:5489
Li L, Davande H, Bedrov D, Smith GD (2007) A molecular dynamics simulation study of C60 fullerenes inside a dimyristoylphosphatidylcholine lipid bilayer. J Phys Chem B 111:4067–4072
Liang L, Kong Z, Kang Z, Wang H, Zhang L, Shen J-W (2016) Theoretical evaluation on potential cytotoxicity of graphene quantum dots. ACS Biomater-Sci Eng. doi:10.1021/acsbiomaterials.6b00390
Lim D-J, Sim M, Oh L, Lim K, Park H (2014) Carbon-based drug delivery carriers for cancer therapy. Arch Pharm Res 37:43–52
Liu Z, Chen K, Davis C, Sherlock S, Cao Q, Chen X, Dai H (2008) Drug delivery with carbon nanotubes for in vivo cancer treatment. Canc Res 68:6652–6660
Marrink SJ, De Vries AH, Mark AE (2004) Coarse grained model for semiquantitative lipid simulations. J Phys Chem B 108:750–760
Marrink SJ, Risselada J, Mark AE (2005) Simulation of gel phase formation and melting in lipid bilayers using a coarse grained model. Chem Phys Lipid 135:223–244
Marrink SJ, Risselada HJ, Yefimov S, Tieleman DP, De Vries AH (2007) The MARTINI force field: coarse grained model for biomolecular simulations. J Phys Chem B 111:7812–7824
Mehdipour N, Bahri K (2013) Mesoscale simulation of water. J Iran Chem Soc 10:1123–1128
Mekaru H, Lu J, Tamanoi F (2015) Development of mesoporous silica-based nanoparticles with controlled release capability for cancer therapy. Adv Drug Deliver Rev 95:40–49
Montellano A, Da Ros T, Bianco A, Prato M (2011) Fullerene C 60 as a multifunctional system for drug and gene delivery. Nanoscale 3:4035–4041
Monticelli L (2012) On atomistic and coarse-grained models for C60 fullerene. J. Chem Theo Comput 8:1370–1378
Monticelli L, Kandasamy SK, Periole X, Larson RG, Tieleman DP, Marrink S-J (2008) The MARTINI coarse-grained force field: extension to proteins. J. Chem Theo Comput 4:819–834
Munoz F, Alici G, Li W (2014) A review of drug delivery systems for capsule endoscopy. Adv Drug Deliver Rev 71:77–85
Mura S, Nicolas J, Couvreur P (2013) Stimuli-responsive nanocarriers for drug delivery. Nature Mater 12:991–1003
Natarajan JV, Nugraha C, Ng XW, Venkatraman S (2014) Sustained-release from nanocarriers: a review. J Control Release 193:122–138
Pattni BS, Chupin VV, Torchilin VP (2015) New developments in liposomal drug delivery. Chem Rev 115:10938–10966
Periole X, Cavalli M, Marrink S-J, Ceruso MA (2009) Combining an elastic network with a coarse-grained molecular force field: structure, dynamics, and intermolecular recognition. J Chem Theo Comput 5:2531–2543
Probst CE, Zrazhevskiy P, Bagalkot V, Gao X (2013) Quantum dots as a platform for nanoparticle drug delivery vehicle design. Adv Drug Deliv Rev 65:703–718
Qiao R, Roberts AP, Mount AS, Klaine SJ, Ke PC (2007) Translocation of C60 and its derivatives across a lipid bilayer. Nano Lett 7:614–619
Raza K et al (2015) C 60-fullerenes for delivery of docetaxel to breast cancer cells: a promising approach for enhanced efficacy and better pharmacokinetic profile. Int J Pharma 495:551–559
Risselada HJ, Marrink SJ (2008) The molecular face of lipid rafts in model membranes. Proc. Nat Acad Sci 105:17367–17372
Rossi G, Barnoud J, Monticelli L (2013) Partitioning and solubility of C60 fullerene in lipid membranes. Phys Scr 87:058503
Saunders MG, Voth GA (2013) Coarse-graining methods for computational biology. Annu Rev Biophys 42:73–93
Shi J et al (2013) PEI-derivatized fullerene drug delivery using folate as a homing device targeting to tumor. Biomaterials 34:251–261
Shimizu K, Kubota R, Kobayashi N, Tahara M, Sugimoto N, Nishimura T, Ikarashi Y (2013) Cytotoxic effects of hydroxylated fullerenes in three types of liver cells. Materials 6:2713–2722
Sridhar A, Srikanth B, Kumar A, Dasmahapatra AK (2015) Coarse-grain molecular dynamics study of fullerene transport across a cell membrane. J Chem Phys 143:024907
Sultana S, Khan MR, Kumar M, Kumar S, Ali M (2013) Nanoparticles-mediated drug delivery approaches for cancer targeting: a review. J Drug Target 21:107–125
Sundar S, Kundu J, Kundu SC (2016) Biopolymeric nanoparticles. Sci. Technol. Adv. Mater
Thakare VS, Prendergast DA, Pastorin G, Jain S (2015) Carbon-based nanomaterials for targeted drug delivery and imaging. In: Targeted drug delivery: concepts and design. Springer, Berlin Heidelberg New York, pp. 615–645
Trpkovic A, Todorovic-Markovic B, Trajkovic V (2012) Toxicity of pristine versus functionalized fullerenes: mechanisms of cell damage and the role of oxidative stress. Arch Toxicol 86:1809–1827
Wang AZ, Langer R, Farokhzad OC (2012) Nanoparticle delivery of cancer drugs. Annu Rev Med 63:185–198
Wilczewska AZ, Niemirowicz K, Markiewicz KH, Car H (2012) Nanoparticles as drug delivery systems. Pharmacol Rep 64:1020–1037
Wong BS, Yoong SL, Jagusiak A, Panczyk T, Ho HK, Ang WH, Pastorin G (2013) Carbon nanotubes for delivery of small molecule drugs. Adv Drug Deliv Rev 65:1964–2015
Wong-Ekkabut J, Baoukina S, Triampo W, Tang IM, Tieleman DP, Monticelli L (2008) Computer simulation study of fullerene translocation through lipid membranes. Nat Nanotechnol 3:363–368
Xing G et al (2004) Influences of structural properties on stability of fullerenols. J Phys Chem B 108:11473–11479
Yin Q, Shen J, Zhang Z, Yu H, Li Y (2013) Reversal of multidrug resistance by stimuli-responsive drug delivery systems for therapy of tumor. Adv Drug Deliv Rev 65:1699–1715
Yun Y, Cho YW, Park K (2013) Nanoparticles for oral delivery: targeted nanoparticles with peptidic ligands for oral protein delivery. Adv Drug Delive Rev 65:822–832
Zabihi Z, Araghi H (2015) Formation of nanopore in a suspended graphene sheet with argon cluster bombardment: a molecular dynamics simulation study. Nucl Instrum Meth B 343:48–51
Zhang S, Chu Z, Yin C, Zhang C, Lin G, Li Q (2013a) Controllable drug release and simultaneously carrier decomposition of SiO2-drug composite nanoparticles. J Am Chem Soc 135:5709–5716
Zhang Y, Chan HF, Leong KW (2013b) Advanced materials and processing for drug delivery: the past and the future. Adv Drug Deliv Rev 65:104–120
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This study was funded by the National Natural Science Foundation of China (grant no. 21503186, 21403049, and 21674032) and Zhejiang Provincial Natural Science Foundation of China (grant no. LY14B030008).
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Liang, L., Kang, Z. & Shen, JW. Translocation mechanism of C60 and C60 derivations across a cell membrane. J Nanopart Res 18, 333 (2016). https://doi.org/10.1007/s11051-016-3647-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-016-3647-z