Abstract
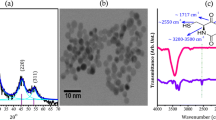
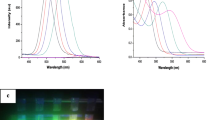
Highly fluorescent nanoparticles, or quantum dots, have multiple applications in biology and biomedicine; however, in most cases, it is necessary to functionalize them to enhance their biocompatibility and selectivity. Generally, functionalization is performed after nanoparticle synthesis and involves the use of molecules or macromolecules having two important traits: specific biological activity and functional groups that facilitate nanoparticle capping (i.e. atom–atom interaction). For this reason, we carried out a simple protocol for the chemical synthesis of cadmium telluride quantum dots capped with glutathione, and we then functionalized these nanoparticles with the amphipathic peptide CLPFFD. This peptide attaches selectively to β-Amyloid fibres, which are involved in Alzheimer’s disease. Our results show that the optical properties of the quantum dots are not affected by functionalization with this peptide. Infrared spectra showed that cadmium telluride quantum dots were functionalized with the peptide CLPFFD. In addition, no significant differences were observed between the surface charge of the quantum dots with or without CLPFFD and the nanocrystal size calculated for HR-TEM was 4.2 nm. Finally, our results show that quantum dots with CLPFFD are stable and that they resulted in a significantly reduced cytotoxicity with respect to that induced by quantum dots not conjugated with the peptide. Moreover, the results show that the CLPFFD-functionalized nanoparticles bind to β-Amyloid fibres.





Similar content being viewed by others
References
Adura C et al (2013) Stable conjugates of peptides with gold nanorods for biomedical applications with reduced effects on cell viability. ACS Appl Mater Interfaces 5:4076–4085. doi:10.1021/am3028537
Araya E, Olmedo I, Bastus NG, Guerrero S, Puntes VF, Giralt E, Kogan MJ (2008) Gold Nanoparticles and microwave irradiation inhibit β-Amyloid amyloidogenesis. Nanoscale Res Lett 3:435–443. doi:10.1007/s11671-008-9178-5
Bang JH, Kamat PV (2009) Quantum dot sensitized solar cells. A tale of two semiconductor nanocrystals: CdSe and CdTe. ACS Nano 3:1467–1476. doi:10.1021/nn900324q
Bieschke J, Siegel SJ, Fu Y, Kelly JW (2008) Alzheimer’s Aβ peptides containing an isostructural backbone mutation afford distinct aggregate morphologies but analogous cytotoxicity. Evidence for a common low-abundance toxic structure(s)? Biochemistry 47:50–59. doi:10.1021/bi701757v
Bradburne CE et al (2013) Cytotoxicity of quantum dots used for in vitro cellular labeling: role of qd surface ligand, delivery modality, cell type, and direct comparison to organic fluorophores. Bioconjug Chem 24:1570–1583. doi:10.1021/bc4001917
Bruchez M, Moronne M, Gin P, Weiss S, Alivisatos AP (1998) Semiconductor nanocrystals as fluorescent biological labels. Science 281:2013–2016. doi:10.1126/science.281.5385.2013
Califano M (2015) Origins of photoluminescence decay kinetics in CdTe colloidal quantum dots. ACS Nano 9:2960–2967. doi:10.1021/nn5070327
Chan WCW, Nie S (1998) Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 281:2016–2018. doi:10.1126/science.281.5385.2016
Díaz V et al (2012) Spectroscopic properties and biocompatibility studies of cdte quantum dots capped with biological thiols. Sci Adv Mater 4:609–618
Dumas EM, Ozenne V, Mielke RE, Nadeau JL (2009) Toxicity of CdTe quantum dots in bacterial strains. IEEE Trans Nanobiosci 8:56–64
Erbo Y, Dan L, Shaojun G, Shaojun D, Jin W (2008) Synthesis and bio-imaging application of highly luminescent mercaptosuccinic acid-coated CdTe nanocrystals. PLoS One 3:e2222–e2227
Fadel TR, Steevens JA, Thomas TA, Linkov I (2015) The challenges of nanotechnology risk management. Nano Today 10:6–10. doi:10.1016/j.nantod.2014.09.008
Faraon A, Englund D, Fushman I, Vučković J, Stoltz N, Petroff P (2007) Local quantum dot tuning on photonic crystal chips. Appl Phys Lett 90:213110. doi:10.1063/1.2742789
Guerrero S et al (2010) Improving the brain delivery of gold nanoparticles by conjugation with an amphipathic peptide. Nanomedicine 5:897–913. doi:10.2217/nnm.10.74
Guerrero S et al (2012) Synthesis and in vivo evaluation of the biodistribution of a 18F-labeled conjugate gold-nanoparticle-peptide with potential biomedical application. Bioconjug Chem 23:399–408. doi:10.1021/bc200362a
Hardman R (2006) A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ Health Perspect 114:165–172. doi:10.1289/ehp.8284
Hoshino A et al (2004) Physicochemical properties and cellular toxicity of nanocrystal quantum dots depend on their surface modification. Nano Lett 4:2163–2169. doi:10.1021/nl048715d
Huang Y-Y et al. (2012) Can nanotechnology potentiate photodynamic therapy? vol 1. doi:10.1515/ntrev-2011-0005
Jameson LP, Smith NW, Dzyuba SV (2012) Dye-binding assays for evaluation of the effects of small molecule inhibitors on amyloid (Aβ) self-assembly. ACS Chem Neurosci 3:807–819. doi:10.1021/cn300076x
Kairdolf BA, Smith AM, Stokes TH, Wang MD, Young AN, Nie S (2013) Semiconductor quantum dots for bioimaging and biodiagnostic applications. Ann Rev Anal Chem 6:143–162. doi:10.1146/annurev-anchem-060908-155136
Kogan MJ et al (2006) Nanoparticle-mediated local and remote manipulation of protein aggregation. Nano Lett 6:110–115. doi:10.1021/nl0516862
Kongkanand A, Tvrdy K, Takechi K, Kuno M, Kamat PV (2008) Quantum dot solar cells. Tuning photoresponse through size and shape control of CdSe–TiO2 architecture. J Am Chem Soc 130:4007–4015. doi:10.1021/ja0782706
Kuzyniak W et al (2014) Synthesis and characterization of quantum dots designed for biomedical use. Int J Pharm 466:382–389. doi:10.1016/j.ijpharm.2014.03.037
Larson DR, Zipfel WR, Williams RM, Clark SW, Bruchez MP, Wise FW, Webb WW (2003) Water-soluble quantum dots for multiphoton fluorescence imaging in vivo. Science 300:1434–1436. doi:10.1126/science.1083780
Let there be light (2015) Nat Mater 14:453. doi:10.1038/nmat4287
Lewinski N, Colvin V, Drezek R (2008) Cytotoxicity of nanoparticles. Small 4:26–49
Lovrić J, Bazzi HS, Cuie Y, Fortin GRA, Winnik FM, Maysinger D (2005) Differences in subcellular distribution and toxicity of green and red emitting CdTe quantum dots. J Mol Med 83:377–385
Medintz IL, Uyeda HT, Goldman ER, Mattoussi H (2005) Quantum dot bioconjugates for imaging, labelling and sensing. Nat Mater 4:435–446
Olmedo I et al (2008) How changes in the sequence of the peptide CLPFFD-NH2 can modify the conjugation and stability of gold nanoparticles and their affinity for β-amyloid fibrils. Bioconjug Chem 19:1154–1163. doi:10.1021/bc800016y
Osovsky R, Kloper V, Kolny-Olesiak J, Sashchiuk A, Efrat Lifshitz E (2007) Optical Properties of CdTe nanocrystal quantum dots, grown in the presence of Cd0 nanoparticles. J Physic Chem C 111:10841–10847
Patra S, Samanta A (2014) Effect of capping agent and medium on light-induced variation of the luminescence properties of CdTe quantum dots: a study based on fluorescence correlation spectroscopy, steady state and time-resolved fluorescence techniques. J Physic Chem C 118:18187–18196. doi:10.1021/jp5048216
Pérez-Donoso JM et al (2012) Biomimetic. Mild chemical synthesis of cdte-gsh quantum dots with improved biocompatibility PLoS One 7:e30741–e30749
Ramirez-Maureira M, Vargas V, Riveros A, Goulet PJG, Osorio-Román IO (2015) Shell-isolated nanoparticle-enhanced fluorescence (SHINEF) of CdTe quantum dots. Mater Chem Phys 2:351–356
Smith AM, Nie S (2010) Semiconductor Nanocrystals: structure, properties, and band gap engineering. Acc Chem Res 43:190–200. doi:10.1021/ar9001069
Soto C, Kindy MS, Baumann M, Frangione B (1996) Inhibition of alzheimer’s amyloidosis by peptides that prevent β-sheet conformation. Biochem Biophys Res Commun 226:672–680. doi:10.1006/bbrc.1996.1413
Soto C, Sigurdsson EM, Morelli L, Asok Kumar R, Castano EM, Frangione B (1998) [beta]-sheet breaker peptides inhibit fibrillogenesis in a rat brain model of amyloidosis: implications for Alzheimer’s therapy. Nat Med 4:822–826
Stewart S, Fredericks PM (1999) Surface-enhanced Raman spectroscopy of peptides and proteins adsorbed on an electrochemically prepared silver surface. Spectrochim Acta Part A Mol Biomol Spectrosc 55:1615–1640. doi:10.1016/S1386-1425(98)00293-5
Tokuraku K, Marquardt M, Ikezu T (2009) Real-time imaging and quantification of amyloid-β peptide aggregates by novel quantum-dot nanoprobes. PLoS One 4:e8492. doi:10.1371/journal.pone.0008492
Vera AM, Cárcamo JJ, Aliaga AE, Gómez-Jeria JS, Kogan MJ, Campos-Vallette MM (2015) Interaction of the CLPFFD peptide with gold nanospheres. A Raman, surface enhanced Raman scattering and theoretical study. Spectrochimica Acta Part A: Mol Biomol Spectrosc 134:251–256. doi:10.1016/j.saa.2014.06.116
Zhang L, Xu C, Li B (2009) Simple and sensitive detection method for chromium (VI) in water using glutathione-capped CdTe quantum dots as fluorescent probes. Microchim Acta 166:61–68
Acknowledgments
Financial support of this paper was provided by the FONDECYT 3130654; Fondap 15130011 and FONDECYT 1130425 Grants.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Riveros, A.L., Astudillo, J., Vásquez, C.C. et al. Capping biological quantum dots with the peptide CLPFFD to increase stability and to reduce effects on cell viability. J Nanopart Res 18, 230 (2016). https://doi.org/10.1007/s11051-016-3463-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-016-3463-5