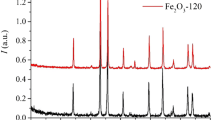
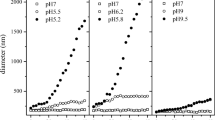
Abstract
Iron oxide nanoparticles are the most used magnetic nanoparticles in biomedical and biotechnological field because of their nontoxicity respect to the other metals. The investigation of iron oxide nanoparticles behaviour in aqueous environment is important for the biological applications in terms of polydispersity, mobility, cellular uptake and response to the external magnetic field. Iron oxide nanoparticles tend to agglomerate in aqueous solutions; thus, the stabilisation and aggregation could be modified tuning the colloids physical proprieties. Surfactants or polymers are often used to avoid agglomeration and increase nanoparticles stability. We have modelled and synthesised iron oxide nanoparticles through a co-precipitation method, in order to study the influence of surfactants and coatings on the aggregation state. Thus, we compared experimental results to simulation model data. The change of Z-potential and the clusters size were determined by Dynamic Light Scattering. We developed a suitable numerical model to predict the flocculation. The effects of Volume Mean Diameter and fractal dimension were explored in the model. We obtained the trend of these parameters tuning the Z-potential. These curves matched with the experimental results and confirmed the goodness of the model. Subsequently, we exploited the model to study the influence of nanoparticles aggregation and stability by Z-potential and external magnetic field. The highest Z-potential is reached up with a small external magnetic influence, a small aggregation and then a high suspension stability. Thus, we obtained a predictive model of Iron oxide nanoparticles flocculation that will be exploited for the nanoparticles engineering and experimental setup of bioassays.












Similar content being viewed by others
References
Baalousha M (2008) Aggregation and surface properties of iron oxide nanoparticles: influence of pH and natural organic matter. Environ Toxicol Chem 27(9):1875–1882
Baalousha M (2009) Aggregation and disaggregation of iron oxide nanoparticles: influence of particle concentration, pH and natural organic matter. Sci Total Environ 407(6):2093–2101. doi:10.1016/j.scitotenv.2008.11.022
Baldassarre F, Vergaro V, Scarlino F, Santis FD, Lucarelli G, della Torre A, Ciccarella G, Rinaldi R, Giannelli G, Leporatti S (2012) Polyelectrolyte capsules as carriers for growth factor inhibitor delivery to hepatocellular carcinoma. Macromol Biosci 12(5):656–665
Barratt G (2003) Colloidal drug carriers: achievements and perspectives. Cell Mol Life Sci 60:21–37
Bautista M, Bomati-Miguel O, Zhao X, Morales M, Gonzàlez-Carreño T, Alejo R, Ruiz-Cabello JD, Veintemillas-Verdaguer S (2004) Comparative study of ferrofluids based on dextran-coated iron oxide and metal nanoparticles for contrast agents in magnetic resonance imaging. Nanotechnology 15:S154S159
Bell G, Levine S, McCartney L (1970) Approximate methods of determining the double-layer free energy of interaction between two charged colloidal spheres. J Colloid Interf Sci 33:335–359
Berry C, Curtis A (2003) Functionalisation of magnetic nanoparticles for applications in biomedicine. J Phys D Appl Phys 36(13):R198–R206
Berry C, Wells S, Charles S, Aitchison G, Curtis A (2004) Cell response to dextran-derivatised iron oxide nanoparticles post internalisation. Biomaterials 25:54055413
Bulte JWM, Kraitchman D (2004) Iron oxide mr contrast agents for molecular and cellular imaging. NMR Biomed 17(7):484–499. doi:10.1002/nbm.924
Butterworth M, Bell S, Armes S, Simpson A (1996) Synthesis and characterization of polypyrrole–magnetite–silica particles. J Colloid Interf Sci 183(1):91–99. doi:10.1006/jcis.1996.0521
Chan D, Henderson D, Barojas J, Homola A (1985) The stability of a colloidal suspension of coated magnetic particles in an aqueous solution. IBM J Res Dev 29(1):1117
Chin CJ, Yiacoumi S, Tsouris C (1998) Shear-induced occulation of colloidal particles in stirred tanks. J Colloid Interf Sci 206(2):532–545
Cho E, Zhang Q, Xia Y (2011) The effect of sedimentation and diffusion on cellular uptake of gold nanoparticles. Nat Nanotechnol 6:385–391
Chung H, Hogg R (1985) Stability criteria for fine-particle dispersions. Coll Surf 15:119–135. doi:10.1016/0166-6622(85)80060-3
Cornell R, Schertmann U (1996) The iron oxides: structure, properties, reactions, occurrence and uses. Mineral Mag 61(408):740–741
Dickson D, L G et al (2012) Dispersion and stability of bare hematite nanoparticles: effect of dispersion tools, nanoparticle concentration, humic acid and ionic strength. Sci Total Environ 419:170–177
Faure B, Salazar-Alvarez G, Bergström L (2011) Hamaker constants of iron oxide nanoparticles. Langmuir 27(14):8659–8664
Fried T, Shemer G, Markovich G (2001) Ordered two-dimensional arrays of ferrite nanoparticles. Adv Mater 13:1158
Frienlander S, Pui D (2004) Emerging issues in nanoparticle aerosol science and technology. J Nanopart Res 6(2):313–320. doi:10.1023/B:NANO.0000034725.89027.6b
Gao J, Li L, Ho P, Mak G, Gu H, Xu B (2006) Combining fluorescent probes and biofunctional magnetic nanoparticles for rapid detection of bacteria in human blood. Adv Mater 18:3145–3148
Gu H, Ho P, Tsang K, Wang L, Xu B (2003) Using biofunctional magnetic nanoparticles to capture vancomycin-resistant enterococci and other gram-positive bacteria at ultralow concentration. J Am Chem Soc 125(51):15,702–15,703
Gu H, Xu K, Xu C, Xu B (2006) Biofunctional magnetic nanoparticles for protein separation and pathogen detection. Chem Commun 9:941–949. doi:10.1039/B514130C
Gupta A, Gupta M (2005) Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26(18):3995–4021. doi:10.1016/j.biomaterials.2004.10.012
Harris LA, Goff JD, Carmichael AY, Riffle JS, Harburn JJ, Pierre TGS, Saunders M (2003) Magnetite nanoparticle dispersions stabilized with triblock copolymers. Chem Mater 15(6):1367–1377. doi:10.1021/cm020994n
Hidber P, Graule T, Gauckler L (1996) Citric acid—a dispersant for aqueous alumina suspensions. J Am Ceram Soc 79(7):1857–1867. doi:10.1111/j.1151-2916.1996.tb08006.x
Honary S, Zahir F (2013) Effect of zeta potential on the properties of nano-drug delivery systems: a review (part 1). Trop J Pharm Res 12(2):255–264
Hu JD, Zevi Y, Kou XM, Xiao J, Wang XJ, Jin Y (2010) Effect of dissolved organic matter on the stability of magnetite nanoparticles under different pH and ionic strength conditions. Sci Total Environ 408(16):3477–3489. doi:10.1016/j.scitotenv.2010.03.033
Huber D (2005) Synthesis, properties, and applications of iron nanoparticles. Small 1(5):482–501. doi:10.1002/smll.200500006
Ito A, Tanaka K, Honda H, Abe S, Yamaguchi H, Kobayashi T (2003) Complete regression of mouse mammary carcinoma with a size greater than 15 mm by frequent repeated hyperthermia using magnetite nanoparticles. J Biosci Bioeng 96(4):364–369. doi:10.1016/S1389-1723(03)90138-1
Ito A, Hayashida M, Honda H, Hata K, Kagami H, Ueda M, Kobayashi T (2004a) Construction and harvest of multilayered keratinocyte sheets using magnetite nanoparticles and magnetic force. Tissue Eng 10:873–880
Ito A, Takizawa Y, Honda H, Kagami HHH, Ueda M, Kobayashi T (2004b) Tissue engineering using magnetite nanoparticles and magnetic force: heterotypic layers of cocultured hepatocytes and endothelial cells. Tissue Eng 10:833840
Ito A, Hibino E, Kobayashi C, Terasaki H, Kagami H, Ueda M, Kobayashi T, Honda H (2005) Construction and delivery of tissue-engineering human retinal pigment epithelial cell sheets using magnetite nanoparticles and magnetic force. Tissue Eng 11:489–496
Ito A, Honda H, Kobayashi T (2006) Cancer immunotherapy based on intracellular hyperthermia using magnetite nanoparticles: a novel concept of heat-controlled necrosis with heat shock protein expression. Cancer Immunol Immunother 55(3):320–328. doi:10.1007/s00262-005-0049-y
Jacobson M (2005) Fundamentals of atmospheric modeling, 2nd edn. Cambridge University Press, Cambridge
Jiang Q, Logan B (1991) Fractal dimensions of aggregates determined from steady-state size distributions. Environ Sci Technol 25(12):2031–2038. doi:10.1021/es00024a007
Kato H, Suzuki M, Fujita K, Horie M, Endoh S, Yoshida Y, Iwahashi H, Takahashi K, Nakamura A, Kinugasa S (2009) Reliable size determination of nanoparticles using dynamic light scattering method for in vitro toxicology assessment. Toxicol In Vitro 23(5):927–934. doi:10.1016/j.tiv.2009.04.006
Kell A, Stewart G, Ryan S, Peytavi R, Boissinot M, Huletsky A, Bergeron M, Simard B (2008) Vancomycin-modified nanoparticles for efficient targeting and preconcentration of gram-positive and gram-negative bacteria. ACS Nano 2(9):1777–1788. doi:10.1021/nn700183g
Kendall K, Kendall M, Rehfeldt F (2011a) Adhesion of nanoparticles. Adhesion of cells. Viruses and nanoparticles. Springer, Netherlands
Kendall K, Kendall M, Rehfeldt F (2011b) Modelling nanoparticle, virus and cell adhesion. Adhesion of cells. Viruses and nanoparticles. Springer, Netherlands
Kusters K (1991) The influence of turbulence on aggregation of small particles in agitated vessels. PhD thesis, Eindhoven University of Technology, The Netherlands
Larsen B, Haag M, Serkova N, Shroyer K, Stoldt C (2008) Controlled aggregation of superparamagnetic iron oxide nanoparticles for the development of molecular magnetic resonance imaging probes. Nanotechnology 19(26):265102, http://stacks.iop.org/0957-4484/19/i=26/a=265102
Laurent S, Dutz S, Häfeli U, Mahmoudi M (2011) Magnetic fluid hyperthermia: focus on superparamagnetic iron oxide nanoparticles. Adv Colloid Interf Sci 166(1–2):8–23. doi:10.1016/j.cis.2011.04.003
Leeuwenburgh S, Ana I, Jansen J (2010) Sodium citrate as an effective dispersant for the synthesis of inorganic–organic composites with a nanodispersed mineral phase. Acta Biomater 6(3):836–844. doi:10.1016/j.actbio.2009.09.005
Leroux J (2007) Injectable nanocarriers for biodetoxification. Nat Nanotechnol 2:679–684
Li Z, Wei L, Gao M, Lei H (2005) One-pot reaction to synthesize biocompatible magnetite nanoparticles. Adv Mater 17(8):1001–1005. doi:10.1002/adma.200401545
Lübbe A, Bergemann C, Riess H, Schriever F, Reichardt P, Possinger K, Matthias M, Dörken B, Herrmann F, Gürtler R, Hohenberger P, Haas N, Sohr R, Sander B, Lemke AJ, Ohlendorf D, Huhnt W, Huhn D (1996) Clinical experiences with magnetic drug targeting: a phase i study with 4-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Res 56(20):4686–4693, http://cancerres.aacrjournals.org/content/56/20/4686.abstract
Mancarella S, Greco V, Baldassarre F, Vergara D, Maffia M, Leporatti S (2015) Polymer-coated magnetic nanoparticles for curcumin delivery to cancer cells. Macromol Biosci. doi:10.1002/mabi.201500142
Mendelev V, Ivanov A (2005) Magnetic properties of ferrofluids: an influence of chain aggregates. J Magn Magn Mater 289:211–214. doi:10.1016/j.jmmm.2004.11.061
Mikhaylova M, Kim D, Bobrysheva N, Osmolowsky M, Semenov V, Tsakalakos T, Muhammed M (2004) Superparamagnetism of magnetite nanoparticles: dependence on surface modification. Langmuir 20(6):2472–2477. doi:10.1021/la035648e
Mornet S, Vasseur S, Grasset F, Duguet E (2004) Magnetic nanoparticle design for medical diagnosis and therapy. J Mater Chem 14:2161–2175. doi:10.1039/B402025A
Neuberger T, Schöpf B, Hofmann H, Hofmann M, von Rechenberg B (2005) Superparamagnetic nanoparticles for biomedical applications: possibilities and limitations of a new drug delivery system. J Magn Magn Mater 293(1):483–496. doi:10.1016/j.jmmm.2005.01.064 (proceedings of the Fifth International Conference on Scientific and Clinical Apllications of Magnetic Carriers)
Nobuto H, Sugita T, Kubo T, Shimose S, Yasunaga Y, Murakami T, Ochi M (2004) Evaluation of systemic chemotherapy with magnetic liposomal doxorubicin and a dipole external electromagnet. Int J Cancer 109(4):627–635. doi:10.1002/ijc.20035
Papaefthymiou G (2009) Nanoparticle magnetism. Nano Today 4(5):438–447, doi:10.1016/j.nantod.2009.08.006, http://www.sciencedirect.com/science/article/pii/S1748013209000929
Ponder S, Darab J, Mallouk T (2000) Remediation of cr(vi) and pb(ii) aqueous solutions using supported, nanoscale zero-valent iron. Environ Sci Technol 34(12):2564–2569. doi:10.1021/es9911420
Roca A, Veintemillas-Verdaguer S, Port M, Robic C, Serna C, Morales M (2009) Effect of nanoparticle and aggregate size on the relaxometric properties of mr contrast agents based on high quality magnetite nanoparticles. J Phys Chem B 113(19):7033–7039. doi:10.1021/jp807820s
Rolliè S, Briesen J, Sundmacher K (2009) Discrete bivariate population balance modelling of heteroaggregation processes. J Colloid Interf Sci 336(2):551–564. doi:10.1016/j.jcis.2009.04.031
Ruysschaert T, Paquereau L, Winterhalter M, Fournier D (2006) Stabilization of liposomes through enzymatic polymerization of dna. Nano Lett 6(12):2755–2757. doi:10.1021/nl061724x
Safarik I, Safarikova M (2004) Magnetic techniques for the isolation and purification of proteins and peptides. Biomagn Res Technol 2:7
Saffman P, Turner J (1956) On the collision of drops in turbulent clouds. J Fluid Mech 1:16–30. doi:10.1017/S0022112056000020
Saiyed Z, Telang S, Ramchand C (2003) Application of magnetic techniques in the field of drug discovery and biomedicine. Biomagn Res Technol 1:2
Saleh N, Phenrat T, Sirk K, Dufour B, Ok J, Sarbu T, Matyjaszewski K, Tilton R, Lowry G (2005) Adsorbed triblock copolymers deliver reactive iron nanoparticles to the oil/water interface. Nano Lett 5(12):2489–2494. doi:10.1021/nl0518268
Sayes C, Reed K, Warheit DB (2007) Assessing toxicity of fine and nanoparticles: comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicol Sci 97(1):163–180, doi:10.1093/toxsci/kfm018, http://toxsci.oxfordjournals.org/content/97/1/163.abstract, http://toxsci.oxfordjournals.org/content/97/1/163.full.pdf+html
Selomulya C, Bushell G, Amal R, Waite T (2003) Understanding the role of restructuring in flocculation: the application of a population balance model. Chem Eng Sci 58(2):327–338, doi:10.1016/S0009-2509(02)00523-7, http://www.sciencedirect.com/science/article/pii/S0009250902005237
Shafi K, Ulman A, Yan X, N-LYang, Estournés C, White H, Rafailovich M (2001) Sonochemical synthesis of functionalized amorphous iron oxide nanoparticles. Langmuir 17(16):5093–5097. doi:10.1021/la010421+
Shen L, Laibinis P, Hatton T (1999) Bilayer surfactant stabilized magnetic fluids: synthesis and interactions at interfaces. Langmuir 15:447–453
Shinkai M, Le B, Honda H, Yoshikawa K, Shimizu K, Saga S, Wakabayashi T, Yoshida J, Kobayashi T (2001) Targeting hyperthermia for renal cell carcinoma using human mn antigen-specific magnetoliposomes. Jpn J Cancer Res 92:1138–1145
Smoluchowski M (1916) Drei vortrge ber diffusion, brownsche molekularbewegung und koagulation von kolloidteilchen. JPhys Zeit 17:557571
Sousa M, Tourinho F, Depeyrot J, da Silva G, Lara M (2001) New electric double-layered magnetic fluids based on copper, nickel, and zinc ferrite nanostructures. J Phys Chem B 105(6):1168–1175. doi:10.1021/jp0039161
Spielman L (1970) Viscous interactions is brownian coagulation. J Colloid Interf Sci 33(4):562–570
Sun YP, q Li X, Cao J, x Zhang W, Wang H (2006) Characterization of zero-valent iron nanoparticles. Adv Colloid Interf Sci 120(1–3):47 56. doi:10.1016/j.cis.2006.03.001
Suzuki M, Shinkai M, Honda H, Kobayashi T (2003) Anticancer effect and immune induction by hyperthermia of malignant melanoma using magnetite cationic liposomes. Melanoma Res 13:129–135
Taboada-Serrano P, Chin CJ, Yiacoumi S, Tsouris C (2005) Modeling aggregation of colloidal particles. Curr Opin Colloid Interf Sci 10(3–4):123–132
Tanimoto A, Oshio K, Suematsu M, Pouliquen D, Stark D (2001) Relaxation effects of clustered particles. J Magn Reson Imaging 14(1):72–77. doi:10.1002/jmri.1153
Tartaj P, del Puerto Morales M, Veintemillas-Verdaguer S, Gonzàlez-Carreño T, Serna C (2003) The preparation of magnetic nanoparticles for applications in biomedicine. J Phys D Appl Phys 36(13):R182
Tartaj P, del Puerto Morales M, Gonzàlez-Carreño T, Veintemillas-Verdaguer S, Serna C (2005) Advantages in magnetic nanoparticles for biotechnology applications. J Magn Magn Mater 28:290–291
Teeguarden J, Hinderliter P, Orr G, Thrall B, Pounds J (2007) Particokinetics in vitro: dosimetry considerations for in vitro nanoparticle toxicity assessments. Toxicol Sci 95(2):300–312, doi:10.1093/toxsci/kfl165, http://toxsci.oxfordjournals.org/content/95/2/300.abstract
Thomas D, Judd S, Fawcett N (1999) Flocculation modelling: a review. Water Res 33(7):1579–1592
Thünemann A, Schütt D, Kaufner L, Pison U, Möhwald H (2006) Maghemite nanoparticles protectively coated with poly(ethylene imine) and poly(ethylene oxide)-block-poly(glutamic acid). Langmuir 22(5):2351–2357. doi:10.1021/la052990d
Tietze S, Duerr S, Alexiou C (2012) Nanoparticles for cancer therapy using magnetic forces. Nanomedicine 7(3):447–457
Tsouris C, Scott T (1995) Flocculation of paramagnetic particles in a magnetic field. J Colloid Interf Sci 171(2):319–330
Verma A, Stellacci F (2010) Effect of surface properties on nanoparticlecell interactions. Small 6(1):12–21
Vishista K, Gnanam F (2004) Role of deflocculants on the rheological properties of boehmite sol. Mater Lett 58:1576–81
Wan M, Li J (1998) Synthesis and electrical–magnetic properties of polyaniline composites. Polymer Sci 36:2799–2805
Widder K, Senyei A, Scarpelli D (1978) Magnetic microspheres: a model system for site specific drug delivery in vivo. Proc Soc Exp Biol Med 58:141
Wiesner M (1992) Kinetics of aggregate formation in rapid mix. Water Res 26(3):379–387
Ying T, Yacoumi S, Tsouris C (2000) High-gradient magnetically seeded filtration. Chem Eng Sci 55(6):1101–1113
Zhang J, Srivastava R, Misra R (2007) Core–shell magnetite nanoparticles surface encapsulated with smart stimuli-responsive polymer: synthesis, characterization, and lcst of viable drug-targeting delivery system. Langmuir 23(11):6342–6351. doi:10.1021/la0636199
Zhang L, Granick S (2006) How to stabilize phospholipid liposomes (using nanoparticles). Nano Lett 6:694–698
Zhu D, Liu F, Ma L, Liu D, Wang Z (2013) Nanoparticle-based systems for t1-weighted magnetic resonance imaging contrast agents. Int J Mol Sci 14(5):10,591–10,607, doi:10.3390/ijms140510591, http://www.mdpi.com/1422-0067/14/5/10591
Acknowledgments
This research was supported by PON 254/Ric. Potenziamento del “CENTRO RICERCHE PER LA SALUTE DELL’UOMO E DELL’AMBIENTE” Cod. PONa300334. CUP: F81D11000210007. Nanotecnologie molecolari per il rilascio controllato di farmaci/NANO Molecular tEchnologies for Drug delivery NANOMED prot. 2010FPTBSH, CUP: F81J12000380001 and by “POR Calabria FSE 2007/2013—Obiettivo Operativo M2—Sostenere la realizzazione di percorsi individuali di alta formazione per giovani laureati e ricercatori presso organismi di riconosciuto prestigio nazionale e internazionale”.
Author information
Authors and Affiliations
Corresponding author
Appendix A
Appendix A
Hydrodynamic resistance
\(D_\infty\) is the diffusion coefficient in the absence of any interparticle forces given by
where D i and D j are the diffusion coefficients of particles i and j, respectively, \(k_B\) is the Boltzmann constant, T is the temperature, \(\mu\) is the viscosity of the medium. Denoting a function of particle separation \(D_{i,j} = b k_B T\) as the relative diffusion coefficient between particles i and j, we adopted the formulation by Jacobson (2005) for \(\frac{D_\infty }{D_{i,j}}\), since the adaption of Van der Waals/viscous collision correction factor from aerosol particles theory, thus generating a so called viscous-force correction factor to the diffusion coefficient in the continuum regime.
where r is the centre-to-centre distance between two flocs or, generally, aggregations, i.e. interparticle distance. Please, note that b is the relative mobility of the particles; for two spheres moving along their centreline, it can be obtained from the exact solution of Stokes’ equation (Spielman 1970).
Van der Waals interaction
Hamaker’s formula for the London-van der Waals attractive interaction energy Vvdw between spherical particles is
where A is the Hamaker constant, \(k_B\) is the Boltzmann constant, T is the temperature and r is the interparticle distance.
Electrostatic interaction
In the case of thin double layers, symmetrical electrolytes and dimensionless interparticle separations \(\kappa l\) \({>}4\) (where \(\kappa\) is the inverse Debye-Huckel length, a measure of the double-layer thickness), the linear superposition approximation can be used
where z represents the valence of the electrolyte dissociated in the solution, and \(\psi _{0i}\) represents the surface potential of the particle i. It is assumed that the potential of one particle remains undisturbed in the presence of the others. The inverse Debye-Huckel length \(\kappa\) is given by \(\kappa ^{-1}\cong 2.8\times 10^{-8} I^{-0.5}\), where I is the ionic strength of the solution.
This formulation is useful when interparticle distance is greater than 36 nm. For smaller distances, the Derjaguin approximation (Chung and Hogg 1985) can be used
Bell et al. (1970) defined \(V_{{\hbox{el}}}\) according to the following formulation:
Magnetic interaction
Chan et al. (1985) provided a formula for calculating the magnetic interaction
where
and
where \(\mu _0\) is the permeability of free space, \(\chi\) is the magnetic susceptibility, B is the strength of the magnetic field, d is the particle diameter and r is the separation of the particles. But, this formulation does not seem consistent, since it does not incorporate the sphericity of flocs or aggregations. In order to consider this issue, we used the following formulation (Bell et al. 1970) for the x factor:
Rights and permissions
About this article
Cite this article
Baldassarre, F., Cacciola, M. & Ciccarella, G. A predictive model of iron oxide nanoparticles flocculation tuning Z-potential in aqueous environment for biological application. J Nanopart Res 17, 377 (2015). https://doi.org/10.1007/s11051-015-3163-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-015-3163-6