Abstract
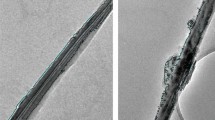
Halloysite nanotubes (HNT) (cylindrical shape with external diameter and length in the range of 30–80 nm and 0.2–1 µm, respectively) were functionalized with 3-aminopropyltriethoxysilane (APTES) from hydroxyl groups by a coupling reaction. Subsequently, maleic anhydride was attached to the APTES moieties to yield carboxylic acid-functionalized HNT. Loading and subsequent release of a model drug molecule diphenhydramine hydrochloride (DPH) on modified and unmodified nanotubes were investigated. Morphology of HNT was studied by electron microscopy. Successful attachment of APTES and carboxylic acid groups to halloysite and drug loading were evaluated by Fourier transform infrared spectroscopy. The amount of surface modification and drug adsorption capacity were calculated via thermogravimetric analysis. The ordered crystal structure of loaded drug was evaluated by X-ray diffraction. UV–Visible spectrophotometer was used to study drug release from modified and unmodified samples. Carboxylated halloysite exhibits higher loading capacity and prolonged release of DPH as compared to that of the natural halloysite.







Similar content being viewed by others
References
Aguzzi C, Viseras C, Cerezo P, Salcedo I, Sanchez-Espejo R, Valenzuela C (2013) Release kinetics of 5-aminosalicylic acid from halloysite. Colloids Surf B Biointerfaces 105:75–80
Ali MS, Ghori M, Rafiuddin S, Khatri AR (2007) A new hydrophilic interaction liquid chromatographic (HILIC) procedure for the simultaneous determination of pseudoephedrine hydrochloride (PSH), diphenhydramine hydrochloride (DPH) and dextromethorphan hydrobromide (DXH) in cough-cold formulations. J Pharm Biomed Anal 43:158–167
Ambrogi V, Fardella G, Grandolini G, Perioli L (2001) Intercalation compounds of hydrotalcite-like anionic clays. Int J Pharm 220:23–32
Choy J, Choi S, Oh J, Park T (2007) Clay minerals and layered double hydroxides for novel biological applications. Appl Clay Sci 36:122–132
Emami SH, Pirbasti ZH, Hasani-Sadrabadi MM, Kordestani SS (2011) The effect of isopropanol addition on enhancement of transdermal controlled release of ibuprofen from ethylene vinyl acetate copolymer membranes. J Appl Polym Sci 122:3048–3054
Eslami H, Solati-Hashjin M, Tahriri M (2009) Effect of fluorine ion addition on structural, thermal, mechanical, solubility and biocompatibility characteristics of hydroxyapatite nanopowders. Adv Appl Ceram 000:1–13
Fejer I, Kata M, Eros I, Dekani I (2002) Interaction of monovalent cationic drugs with montmorillonite. Colloid Polym Sci 280:372–379
Forsgren J, Jämstorp E, Bredenberg S, Engqvist H, Strømme M (2010) A ceramic drug delivery vehicle for oral administration of highly potent opioids. J Pharm Sci 99:219–226
Ghebaur A, Garea SA, Iovu H (2012) New polymer-halloysite hybrid materials—potential controlled drug release system. Int J Pharm 436:568–573
He Q, Yang D, Deng X, Wu Q, Li R, Zhai Y, Zhang L (2013) Preparation, characterization and application of N-2-Pyridylsuccinamic acid-functionalized halloysite nanotubes for solid-phase extraction of Pb(II). Water Res 47:3976–3983
Jinhua W, Xiang Z, Bing Z, Yafei Z, Rui Z, Jindun L, Rongfeng C (2010) Rapid adsorption of Cr (VI) on modified halloysite nanotubes. Desalination 259:22–28
Joo Y, Jeon Y, Lee SU, Sim JH, Ryu J, Lee S, Lee H, Sohn D (2012) Aggregation and stabilization of carboxylic acid functionalized halloysite nanotubes (HNT-COOH). J Phys Chem C 116:18230–18235
Joshi GV, Patel HA, Kevadiya BD, Bajaj HC (2009) Montmorillonite intercalated with vitamin B1 as drug carrier. Appl Clay Sci 45:248–253
Kelly HM, Deasy PB, Ziaka E, Claffey N (2004) Formulation and preliminary in vivo dog studies of a novel drug delivery system for the treatment of periodontitis. Int J Pharm 274:167–183
Kim H, Fassihi R (1997) Application of binary polymer system in drug release rate modulation. 2. Influence of formulation variables and hydrodynamic conditions on release kinetics. J Pharm Sci 86:323–328
Levis SR, Deasy PB (2002) Characterisation of halloysite for use as a microtubular drug delivery. Int J Pharm 243:125–134
Levis SR, Deasy PB (2003) Use of coated microtubular halloysite for the sustained release of diltiazem hydrochloride and propranolol hydrochloride. Int J Pharm 253:145–157
Lvov YM, Price RR (2008) Halloysite nanotubules, a novel substrate for the controlled delivery of bioactive molecules. In: Ruiz-Hitzky E, Ariga K, Lvov YM (eds) Bio-inorganic hybrid nanomaterials. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 419–442
Lvov YM, Shchukin DG, Mohwald HM, Price RR (2008) Halloysite clay nanotubes for controlled release of protective agents. ACS Nano 2:814–820
Mitchell MJ, Chen CS, Ponmudi V, Hughes AD, King MR (2012) E-selectin liposomal and nanotube-targeted delivery of doxorubicin to circulating tumor cells. J Control Release 160:609–617
Nandgude TD, Bhise KS, Gupta VB (2008) Characterization of hydrochloride and tannate salts of diphenhydramine. Indian Journal of Pharmaceutical Sciences 70:482-486
Pan J, Yao H, Xu L, Ou H, Huo P, Li X, Yan Y (2011) Selective recognition of 2,4,6-trichlorophenol by molecularly imprinted polymers based on magnetic halloysite nanotubes composites. J Phys Chem C 115:5440–5449
Papadopoulou V, Kosmidis K, Vlachou M, Macheras P (2006) On the use of the Weibull function for the discernment of drug release mechanisms. Int J Pharm 309:44–50
Peppas N (1985) Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv 60:110
Qi R, Guo R, Zheng F, Liu H, Yu J, Shi X (2013) Controlled release and antibacterial activity of antibiotic-loaded electrospun halloysite/poly(lactic-co-glycolic acid) composite nanofibers. Colloids Surf B Biointerfaces 110:148–155
Siepmann J, Peppas N (2012) Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Deliv Rev 64:163–174
Singh B, Mackinnon IDR (1996) Experimental transformation of kaolinite to halloysite. Clays Clay Miner 44:825–834
Tan D, Yuan P, Annabi-Bergaya F, Yu H, Liu D, Liu H, He H (2013) Natural halloysite nanotubes as mesoporous carriers for the loading of ibuprofen. Microporous Mesoporous Mater 179:89–98
Tan D, Yuan P, Annabi-Bergaya F, Liu D, Wang L, Liu H, He H (2014a) Loading and in vitro release of ibuprofen in tubular halloysite. Appl Clay Sci 96(SI):50–55
Tan D, Yuan P, Annabi-Bergaya F, Liu D, Wang L, Liu H, He H (2014b) Loading and in vitro release of ibuprofen in tubular halloysite. Appl Clay Sci 96:50–55
Veerabadran NG, Price RR, Lvov YM (2007) Clay nanotubes for encapsulation and sustained release of drugs. NANO 2:115–120
Vergaro V, Abdullayev E, Lvov YM, Zeitoun A, Cingolani R, Rinaldi R, Zeitoun A, Leporatti S (2010) Cytocompatibility and uptake of halloysite clay nanotubes. Biomacromolecules 11:820–826
Viseras MT, Aguzzi C, Cerezo P, Viseras C, Valenzuela C (2008) Equilibrium and kinetics of 5-aminosalicylic acid adsorption by halloysite. Microporous Mesoporous Mater 108:112–116
Wang Q, Zhang J, Wang A (2013) Alkali activation of halloysite for adsorption and release of ofloxacin. Appl Surf Sci 287:54–61
Wang Q, Zhang J, Zheng Y, Wang A (2014) Adsorption and release of ofloxacin from acid- and heat-treated halloysite. Colloids Surf B Biointerfaces 113:51–58
Yah WO, Takahara A, Lvov YM (2012a) Selective modification of halloysite lumen with octadecylphosphonic acid: new inorganic tubular micelle. J Am Chem Soc 134:1853–1859
Yah WO, Xu H, Soejima H, Ma W, Lvov Y, Takahara A (2012b) Biomimetic dopamine derivative for selective polymer modification of halloysite nanotube lumen. J Am Chem Soc 134:12134–12137
Yuan P, Southon PD, Liu Z, Green MER, Hook JM, Antill SJ, Kepert CJ (2008) Functionalization of halloysite clay nanotubes by grafting with γ-aminopropyltriethoxysilane. J Phys Chem C 112:15742–15751
Yuan P, Southon PD, Liu Z, Kepert CJ (2012) Organosilane functionalization of halloysite nanotubes for enhanced loading and controlled release. Nanotechnology 23:375705
Zheng JP, Luan L, Wang HY, Xi LF, Yao KD (2007) Study on ibuprofen/montmorillonite intercalation composites as drug release system. Appl Clay Sci 36:297–301
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zargarian, S.S., Haddadi-Asl, V. & Hematpour, H. Carboxylic acid functionalization of halloysite nanotubes for sustained release of diphenhydramine hydrochloride. J Nanopart Res 17, 218 (2015). https://doi.org/10.1007/s11051-015-3032-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-015-3032-3