Abstract
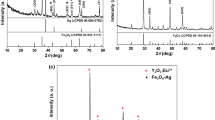
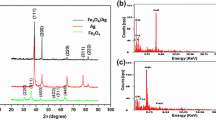
Noble metal and ferromagnetic oxides nanocomposites have attracted great interest because of their optical and magnetic properties. In this paper, we prepared Ag–Fe3O4 nanocomposites by a facile, one-step, and repeatable solvothermal method. The diameter of the as-synthesized nanocomposites was about 250 nm, and the composition of the products was tuned by varying the molar ratio of Ag/Fe. The saturated magnetization of the Ag–Fe3O4 nanocomposites at room temperature gradually decreased with increasing of Ag/Fe molar ratios. The saturated magnetization of the products was 30.6 emu g−1 with the molar ratio of 1:1, which enables them to be easily concentrated from the solution by simply applying a small magnet. These nanocomposites have broad surface plasmon resonance absorption bands from 400 to 600 nm, which are overlapping with the excitation laser of 532 nm. The surface-enhanced Raman spectroscopy (SERS) properties of the nanocomposites were studied using Rhodamine 6G and crystal violet as the target molecules. The correlation of the product composition on SERS was then demonstrated by gradually increasing Ag/Fe molar ratios. So these efficiently and conveniently concentrated products have a great potential in the fields of the biomedical sensitive detection and assay.







Similar content being viewed by others
References
Angeloni L, Smulevich G, Marzocchi MP (1979) Resonance Raman spectrum of crystal violet. J Raman Spectrosc 8(6):305–310
Bao ZY, Dai JY, Lei DY, Wu YC (2013) Maximizing surface-enhanced raman scattering sensitivity of surfactant-free Ag–Fe3O4 nanocomposites through optimization of silver nanoparticle density and magnetic self-assembly. J Appl Phys 114:124305
Bica I (2008) Production of iron nanotubes in plasma. J Ind Eng Chem 14(2):230–235
Bica I (2009) Steady current plasma macro-nanotechnologies. J Ind Eng Chem 15(3):304–315
Deng H, Li XL, Peng Q, Wang X, Chen JP, Li YD (2005) Monodisperse magnetic single-crystal ferrite microspheres. Angew Chem Int Ed 44:2782–2785
Fang JX, Yi Y, Ding BJ, Song XP (2008) A route to increase the enhancement factor of surface enhanced Raman scattering (SERS) via a high density Ag flower-like pattern. Appl Phys Lett 92(13):131115–131117
Fleischmann M, Hendra PJ, McQuillan AJ (1974) Raman spectra of pyridine adsorbed at a silver electrode. Phys Chem Lett 26:163–166
Gan ZB, Zhao AW, Zhang MF, Wang DP, Tao WY, Guo HY, Li D, Li M, Gao Q (2012) A facile strategy for obtaining fresh Ag as SERS active substrates. J Colloid Interface Sci 366(1):23–27
Gühlke M, Selve S, Kneipp J (2012) Magnetic separation and SERS observation of analyte molecules on bifunctional silver/iron oxide composite nanostructures. J Raman Spectrosc 43(9):1204–1207
Guo SJ, Dong SJ, Wang EK (2009) A General route to construct diverse multifunctional Fe3O4/metal hybrid nanostructures. Chem Eur J 15(10):2416–2424
Han SY, Guo QH, Xu MM, Yuan YX, Shen LM, Yao JL, Liu W, Gu RA (2012) Tunable fabrication on iron oxide/Au/Ag nanostructures for surface enhanced Raman spectroscopy and magnetic enrichment. J Colloid Interface Sci 378(1):51–57
Hu JW, Zhang Y, Li JF, Liu Z, Ren B, Sun SG, Tian ZQ (2005) Synthesis of Au@Pd core–shell nanoparticles with controllable size and their application in surface-enhanced Raman spectroscopy. Chem Phys Lett 408(4–6):354–359
Jiang WQ, Zhou YF, Zhang YL, Xuan SH, Gong XL (2012) Superparamagnetic Ag@Fe3O4 core–shell nanospheres: fabrication, characterization and application as reusable nanocatalysts. Dalton Trans 41:4594–4601
Lee PC, Melsel D (1982) Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J Phys Chem 86:3391–3395
Lee SJ, Morrill AR, Moskovits M (2006) Hot spots in silver nanowire bundles for surface-enhanced Raman spectroscopy. J Am Chem Soc 128(7):2200–2201
Liang HY, Li ZP, Wang WZ, Xu YS, Xu HX (2009) Highly surface-roughened “flower-like” silver nanoparticles for extremely sensitive substrates of surface-enhanced Raman scattering. Adv Mater 21(45):4614–4618
Liu XF, Sun CH, Jiang P (2009) Wafer-scale surface-enhanced Raman scattering substrates with highly reproducible enhancement. J Phys Chem C 113(33):14804–14811
Lopes G, Vargas JM, Sharma SK, Beron F, Pirota KR, Knobel M, Rettori C, Zysler RD (2010) Ag–Fe3O4 dimer colloidal nanoparticles: synthesis and enhancement of magnetic properties. J Phys Chem C 114(22):10148–10152
Lv BL, Xu Y, Tian H, Wu D, Sun YH (2010) Synthesis of Fe3O4\SiO2\Ag nanoparticles and its application in surface-enhanced Raman scattering. J Solid State Chem 183(12):2968–2973
Mariotti D, Sankaran RM (2010) Microplasmas for nanomaterials synthesis. J Phys D 43:323001
Nie SM, Emory SR (1997) Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 275:1102–1106
Philip D (2008) Synthesis and spectroscopic characterization of gold nanoparticles. Spectrochim Acta Part A 71(1):80–85
Philip D, Gopchandran KG, Unni C, Nissamudeen KM (2008) Synthesis, characterization and SERS activity of Au–Ag nanorods. Spectrochim Acta Part A 70(4):780–784
Ren B, Lin XF, Yang ZL, Liu GK, Aroca RF, Mao BW, Tian ZQ (2003) Surface-enhanced Raman scattering in the ultraviolet spectral region: UV-SERS on rhodium and ruthenium electrodes. J Am Chem Soc 125(32):9598–9599
Shi L, Lin HL (2011) Preparation of band gap tunable SnO2 nanotubes and their ethanol sensing properties. Langmuir 27:3977–3981
Solla-Gullón J, Gómez R, Aldaz A, Pérez JM (2008) A combination of SERS and electrochemistry in Pt nanoparticle electrocatalysis: promotion of formic acid oxidation by ethylidyne. Electrochem Commun 10(2):319–322
Sun LL, Song YH, Wang L, Guo CL, Sun YJ, Liu ZL, Li Z (2008) Ethanol-induced formation of silver nanoparticle aggregates for highly active SERS substrates and application in DNA detection. J Phys Chem C 112(5):1415–1422
Sun LJ, He J, An SS, Zhang JW, Ren D (2013) Facile one-step synthesis of Ag@Fe3O4 core–shell nanospheres for reproducible SERS substrates. J Mol Struct 1046:74–81
Tian ZQ, Ren B, Wu DW (2002) Surface-enhanced Raman scattering: from noble to transition metals and from rough surfaces to ordered nanostructures. J Phys Chem B 106(37):9463–9483
Tian Y, Chen LJ, Zhang J, Ma ZF, Song CN (2012) Bifunctional Au-nanorod@Fe3O4 nanocomposites: synthesis, characterization, and their use as bioprobes. J Nanopart Res 14(7):998–1008
Wei Y, Bishop KJM, Kim J, Soh S, Grzybowski BA (2009) Making use of bond strength and steric hindrance in nanoscale “synthesis”. Angew Chem Int Ed 48(50):9477–9480
Wheeler DA, Adams SA, Lopez- Luke T, Torres-Castro A, Zhang JZ (2012) Magnetic Fe3O4–Au core–shell nanostructures for surface enhanced Raman scattering. Ann Phys 524(11):670–679
Xu HX, Bjerneld EJ, Kall M, Borjesson L (1999) Spectroscopy of single hemoglobin molecules by surface enhanced Raman scattering. Phys Rev Lett 83(21):4357–4360
Xu C, Xie J, Ho D, Wang C, Kohler N, Walsh EG, Morgan JR, Chin YE, Sun S (2007) Au–Fe3O4 dumbbell nanoparticles as dual-functional probes. Angew Chem Int Ed 47(1):173–176
Xuan SH, Zhou YF, Xu HJ, Jiang WQ, Cham-Fai Leung K, Gong XL (2011) One step method to encapsulate nanocatalysts within Fe3O4 nanoreactors. J Mater Chem 21:15398–15404
Yang Y, Matsubara S, Xiong L, Hayakawa T, Nogami M (2007) Solvothermal synthesis of multiple shapes of silver nanoparticles and their SERS properties. J Phys Chem C 111(26):9095–9104
Yang LB, Bao ZY, Wu YC, Liu JH (2012) Clean and reproducible SERS substrates for high sensitive detection by solid phase synthesis and fabrication of Ag-Coated Fe3O4 microspheres. J Raman Spectrosc 43(7):848–856
Zhang DH, Li GD, Lia JX, Chen JS (2008) One-pot synthesis of Ag–Fe3O4 nanocomposite: a magnetically recyclable and efficient catalyst for epoxidation of styrene. Chem Commun 29:3414–3416
Zhang MF, Zhao AW, Guo HY, Wang DP, Gan ZB, Sun HH, Li D, Li M (2011a) Green synthesis of rosettelike silver nanocrystals with textured surface topography and highly efficient SERS performances. CrystEngComm 13:5709–5717
Zhang MF, Zhao AW, Sun HH, Guo HY, Wang DP, Gan ZB, Li D, Tao WY (2011b) Rapid, large-scale, sonochemical synthesis of 3D nanotextured silver microflowers as highly efficient SERS substrates. J Mater Chem 21:18817–18824
Zhang H, Harpster MH, Wilson WC, Johnson PA (2012a) Surface-enhanced Raman scattering detection of DNAs derived from virus genomes using Au-coated paramagnetic nanoparticles. Langmuir 28(8):4030–4037
Zhang YX, Ding HL, Liu YY, Pan SS, Luo YY, Li GH (2012b) Facile one-step synthesis of plasmonic/magnetic core/shell nanostructures and their multifunctionality. J Mater Chem 22:10779–10786
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grants 61378038), the National Basic Research Program of China (2011CB302103), and the State Key Laboratories of Transducer Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, H., Zhao, A., Gao, Q. et al. One-step synthesis of Ag–Fe3O4 nanocomposites and their SERS properties. J Nanopart Res 16, 2538 (2014). https://doi.org/10.1007/s11051-014-2538-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-014-2538-4