Abstract
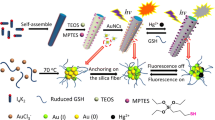
In this study, we design a nanoassembly-based chemosensor possessing the fluorescence in the visible region which comes into play for analyte detection in aqueous medium. Here, Mercaptopropionic acid-functionalised nanophosphor (Gd2O3:Eu @ MPA) acts as donor, and the Cysteamine functionalised gold nanorod (AuNR @ Cysteamine) acts as the acceptor molecule. The working principle of this nanoassembly is the FRET phenomenon which happens between nanophosphors and gold nanorods through amine-carboxyl attractive interactions (Turn-Off) followed by the Meisenheimer complex formation between –NO2 groups of TNT and primary amines of the Cysteamine functionalised AuNR (Turn-On) which gives corresponding fluorescent signals in the visible regions. The fluorescence turn-on is immediate, and the limit of detection is as low as 11.88 x 10−9 M. The above-mentioned phenomena were substantiated using the UV–Visible, Photoluminescence, and Time-Correlated Single Photon Counting spectroscopic techniques. The size, morphology, particle interactions, charge, and functionalisations were substantiated through TEM, DLS, Zeta potential, and FTIR techniques. The size variations happened to the AuNR in three different stages are evident from the TEM images. (i) when AuNR (Gold nanorod) is present alone, i.e. LnNp and TNT free system, the average size of AuNR was 15.17 nm (ii) When LnNp (Lanthanide Nanophosphor) was added (attached), i.e. AuNR + LnNp involved state, the average size of AuNR was increased to 23.05 nm (iii) When TNT was introduced to AuNR + LnNp system (Analyte attachment and LnNp detachment happening state) i.e. AuNR + LnNp + TNT involved state, the average size of AuNR was decreased to16.3 nm as it was in its pristine form. The same trend was obtained for the DLS measurements.












Similar content being viewed by others
References
Aditya Narayanan, Varnavski OP, Swager TM, Theodore Goodson (2008) Multiphoton fluorescence quenching of conjugated polymers for TNT Detection. J Phys Chem C 112:881–884
Chandra S, Doran J, McCormack SJ, Kennedy M, Chatten AJ (2012) Enhanced quantum dot emission for luminescent solar concentrators using plasmonic interaction. Sol Energy Mater Sol Cells 98:385–390
Changmin Deng, Pei Gong, Qingguo He, Jiangong Cheng, Chao He, Liqi Shi, Defeng Zhu, Tong Lin (2009) Highly Fluorescent TPA-PBPV nanofibers with amplified sensing response to TNT. Chem Phys Lett 483:219–223
Colton RJ, Russell JN (2003) Making the world a safer place. Science 299:1324–1325
Daming Gao, Zhenyang Wang, Bianhua Liu, Lin Ni, Minghong Wu, Zhongping Zhang (2008) Resonance Energy Transfer-Amplifying Fluorescence Quenching at the surface of silica Nanoparticles towards ultra sensitive detection of TNT. Anal Chem 80:8545–8553
Doose S, Neuweiler H, Sauer M (2005) A close look at fuorescence quenching of organic dyes by tryptophan. Chem Phys Chem 6:2277–2285
Dosev D, Kennedy IM, Godlewski M, Gryczynski I, Tomsia K, Goldys EM (2006) Fluorescence upconversion in Sm-doped Gd2O3. Appl Phys Lett 88:11906–11909
Ferrer E, de Leon M, Hernandez-Rivera SP (2005) Nanoscaled science and engineering for trace explosive sensing: The effect of TNT concentration in the fluorescence emission of CdS quantum dots. Abstracts of papers of the American Chemical Society-230: U267-U267.
Gai S, Yang P, Wang D, Li C, Niu Na, Hea F, Lia X (2011) Monodisperse Gd2O3:Ln (Ln = Eu3 + , Tb3 + , Dy3 + , Sm3 + , Yb3 +/Er3 + , Yb3 +/Tm3 + , and Yb3 +/Ho3 +) nanocrystals with tunable size and multicolor luminescent properties. Cryst Eng Comm 13:5480–5487
Gao F, Cui P, Chen X, Ye Q, Li M, Wang L (2011) An efficient phosphorescence energy transfer between quantum dots and carbon nanotubes for ultrasensitive turn-on detection of DNA. Analyst 136:3973–3980
Germain ME, Knapp MJ (2009) Optical explosives detection: from color changes to fluorescence turn-on. Chem Soc Rev 38:2543–2555
Haixia Zhang, Lijuan Feng, Bingxin Lin, Cuiyan Tong, Changli Lu (2014) Conjugation of PPV functionalized mesoporous silica nanoparticles with graphene oxide for facile and sensitive fluorescence detection of TNT in water through FRET. Dyes Pigm 101:122–129
Hallowell SF (2001) Raman and Infrared Microspectroscopy of the High Explosives TNT, PETN, and RDX heated to their Melting Point and Beyond. Talanta 54:447–458
Hodak JH, Henglein A, Hartland GV (2000) Electron-phonon coupling dynamics in very small between 2 and 8 nm diameter Au nanoparticles. J Chem Phys 112:5942–5947
Jehuda Y, Johonson VJ, Bernier R, Yost U, Mayfield AR, Mahone TH, Vorbeck WC (2005) Reactions in the mass spectrometry of a hydride meisenheimer complex of 2,4,6-trinitrotoluene (TNT). J Mass Spectrom 30:715–722
Kolla P (1997) The Application of Analytical Methods to the Detection of Hidden Explosives and Explosive Devices. Angew Chem 36:800–811
Kulakovich O, Strekal N, Yaroshevich A, Maskevich S (2002) Enhanced Luminescence of CdSe Quantum Dots on Gold Colloids. Nano Lett 2:1449–1452
Lakowicz JR (2006) Principles of Fluorescence Spectroscopy. Springer-Verlag, Berlin Heidelberg
Lijuan Feng, Chungn Wang, Zhonglin Ma, Changli Lu (2013) 8-Hydroxyquinoline functionalized ZnS nanoparticles capped with amine groups: a fluorescent nanosensor for the facile and sensitive detection of TNT through fluorescence resonance energy transfer. Dyes Pigm 97:84–91
Link S, El-Sayed MA (1999) Spectral Properties and Relaxation Dynamics of Surface Plasmon Electronic Oscillations in Gold and Silver Nanodots and Nanorods. J Phys Chem B 103:8410–8426
Moore DS (2004) Instrumentation for trace detection of high explosives. Rev Sci Instrum 75:2499–2512
Murphy CJ, Thompson LB, Alkilany AM, Sisco PN, Boulos SP, Sivapalan ST, Yang JA, Chernak DJ, Huang J (2010) The Many Faces of Gold Nanorods. J Phys Chem Lett 1:2867–2875
Nikobakht B, El-Sayed MA (2003) Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem Mater 15:1957–1962
Pandya Alok, Goswami Heena, Lodha Anand, Menon Shobhana K (2012) A novel nanoaggregation detection technique of TNT using selective and ultrasensitive nanocurcumin as a probe. Analyst 137:1771–1774
Qian JJ, Qiu LG, Wang YM, Yuan YP, Xie AJ, Shen YH (2014) Fabrication of magnetically separable fluorescent terbium-based MOF nanoparticles for highly selective trace level detection of TNT. Dalton Trans 43:3978–3983
Renyong Tu, Bianhua Lin, Zhenyag Wang, Daming Geo, Fens Wang, Qunling Fang, Zhongping Zhang (2008) Amine capped ZnS-Mn2+ Nanocrystals for Fluorescence Detection of Trace TNT explosive. Anal Chem 80:3458–3465
Rodenas A, Zhou G, Jaque D, Gu M (2009) Photonic Properties of Inverse Opals Fabricated from Lanthanide-Doped LaPO4 Nanocrystals. Adv Mater 21:3883–3888
Sapsford KE, Berti L, Medintz IL (2006) Materials for fluorescence resonance energy transfer analysis: beyond traditional donor-acceptor combinations. Angew Chem 45:4562–4588
Shengyang Tao, Guangtao Li, Jinxiang Yin (2007) Fluorescent nanofibrous membranes for trace detection of TNT vapour. J Mater Chem 17:2730–2736
Singh S, Hazard J (2007) Sensor an effective approach for the detection of explosives. Mater 144:15–28
Steinfeld JI, Wormhoudt J (1998) Explosives detection: a challenge for physical chemistry. Annu Rev Phys Chem 49:203–232
Sun J, Ge J, Liu W, Fan Z, Zhang H, Wang P (2011) Highly sensitive and selective colorimetric visualization of streptomycin in raw milk using Au nanoparticles supramolecular assembly. Chem Commun 47:9888–9890
Swager TM, Esser B (2010) Detection of Ethylene gas by fluorescence Turn-on of a conjugated polymer. Angew Chem 122:9056–9059
Tanner PA, Fu LS, Cheng BM (2009) Spectral Band Shifts in the Electronic Spectra of Rare Earth Sesquioxide Nanomaterials Doped with Europium. J Phys Chem C 113:10773–10779
Thomas KG, Kamat PV (2003) Chromophore-functionalized gold nanoparticles. Acc Chem Res 36:888–898
Tong L, Liu D, Shi Jianhui, Yang Xuwei, Yang Hua (2012) Magnetic and Luminescent properties of Fe3O4 @Y2O3:Eu3+ nanocomposites. J Mater Sci 47:132–137
Walker NR, Linman MJ, Timmers MM, Dean SL, Burkett CM, Lloyd JA, Keelor JD, Baughman BM, Edmiston PL (2007) Selective detection of gas-phase TNT by integrated optical waveguide spectrometry using molecularly imprinted sol-gel sensing films. Anal Chim Acta 593:82–91
Wang C, Irudayaraj J (2010) Multifunctional Magnetic-Optical Nanoparticle Probes for Simultaneous Detection, Separation, and Thermal Ablation of Multiple Pathogens. Small 6:283–289
Wang Ya-qin, Zou Wen-Sheng (2011) 3-Aminopropyl triethoxysilane functionalized manganese doped ZnS quantum dots for room-temperature phosphorescence sensing ultra trace 2,4,6- trinitrotoluene in aqueous solution. Talanta 85:469–475
Woodfin RL (2007) Trace Chemical Sensing of Explosives. John Wiley & Sons, Hoboken
Xia Y, Song L, Zhu C (2011) Turn-On and Near-Infrared Fluorescent Sensing for 2,4,6-Trinitrotoluene Based on Hybrid (Gold Nanorod) − (Quantum Dots) Assembly. Anal Chem 83:1401–1407
Yinon Y (2007) Counterterrorist Detection Techniques of Explosives. Elsevier, Amsterdam
Zhou Dan-Ling, Huang Hong, Zheng Jie-Ning, Chen Jian-Rong, Feng Jiu-Ju, Wang Ai-Jun (2013) Polyinosinic acid-stabilized fluorescent silver nanoclusters for sensitive detection of biological thiols. Anal Methods 5:6076–6080
Zou Wen-Sheng, Sheng Dong, Ge Xin, Qiao Jun-Qin, Lian Hong-Zhen (2011a) Room-Temperature Phosphorescence Chemosensor and Rayleigh Scattering Chemodosimeter Dual-Recognition Probe for 2,4,6-Trinitrotoluene Based on Manganese-Doped ZnS Quantum Dots. Anal Chem 83:30–37
Zou Wen-Sheng, Qiao Jun-Qin, Xin Hu, Ge Xin, Lian Hong-Zhen (2011b) Synthesis in aqueous solution and characterisation of a new cobalt-doped ZnS quantum dot as a hybrid ratiometric chemosensor. Anal Chim Acta 708:134–140
Zou Wen-Sheng, Yang Jin, Yang Ting–Ting, Xin Hu, Lian Hong-Zhen (2012) Magnetic-Room temperature phosphorescent multifunctional nanocomposites as chemosensor for detection and photo-driven enzyme mimetics for degradation of 2,4,6-trinitrotoluene. J Mater Chem 22:4720–4727
Zou Wen-Sheng, Wang Ya-qin, Wang Feng, shao Qun, Zhang Jun, Liu Jin (2013) Selective Fluorescence response and magnetic separation probe for 2,4,6- trinitrotoluene based on iron oxide magnetic nanoparticles. Anal Bioanal Chem 405:4905–4912
Zou Wen-Sheng, Zou Fei-Hua, Shao Qun, Zhang Jun, Wang Ya-qin, Xie Fa-Zhi, Ding Yi (2014) A selective fluorescence resonance energy transfer quenching and resonance light scattering enchancement dual-recognition probe for 2,4,6- trinitrotoluene. J. Photochem. Photobio. A 278:82–88
Acknowledgments
The authors thank the Head of CSIR-NIIST (Pappanamcode), CESS (Thiruvananthapuram), SAIF-IIT (Madras), SCT-IMST (Poojapura), Departments of Bio-Technology & Bio-Chemistry, University of Kerala, Kariavattom campus, (Thiruvananthapuram) for the sophisticated characterization techniques provided for the work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11051_2014_2522_MOESM1_ESM.pptx
Fig. S1. The VSM plot of Gd2O3: Eu@MPA Lanthanide nanophosphor taken at Room Temperature. Fig. S2 The Zeta potential (ξ) distribution curve of Gd2O3: Eu@MPA Lanthanide nanophosphor Fig. S3 The Zeta potential (ξ) distribution curve of AuNR@Cysteamine. Fig. S4 FT-IR spectrum of MPA modified LnNP (Gd2O3: Eu@MPA) Fig. S5 FT-IR spectrum of Cysteamine modified AuNR-626 Fig. S6 The DLS size distribution of individual AuNR @ cystemine system Fig. S7 The DLS size distribution of LnNP @MPA: AuNR @ cystemine system Fig. S8 The DLS size distribution of LnNP @MPA: AuNR @ cystemine + TNT system (PPTX 529 kb)
Rights and permissions
About this article
Cite this article
Praveen, G.L., Lekha, G.M., Visakh, V.M. et al. Lanthanide magneto-luminescent and plasmonic (Gd2O3:Eu@AuNR) nanoassembly for the turn-on fluorescence detection of nitro aromatic compound. J Nanopart Res 16, 2522 (2014). https://doi.org/10.1007/s11051-014-2522-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-014-2522-z