Abstract
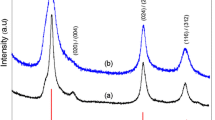
Samples of AgInS2 and CuInS2 nanoparticles were synthesized by hot-injection method at 270 °C using 1-dodecanethiol (DT) and elemental sulphur (S) as sulphide precursors, and oleylamine as reaction medium and surfactant. Composition, crystal structure, and particle size of obtained materials were tracked by XRD and TEM/HRTEM measurements. It was shown that, due to its dual role as sulphur source and surfactant, DT drastically slows formation of desired material. Samples obtained with DT even after 4 h of reaction have traces of intermediary compound (β-In2S3), whereas in samples synthesized with elemental S these traces are less pronounced. The growth mechanism and influence of each reaction step are discussed in detail.








Similar content being viewed by others
References
Abazović ND, Čomor MI, Mitrić MN, Piscopiello E, Radetić T, Janković IA, Nedeljković JM (2012) Ligand mediated synthesis of AgInSe2 nanoparticles with tetragonal/orthorhombic crystal phases. J Nanopart Res 14:810. doi:10.1007/s11051-012-0810-z
Abdelhady AL, Malik MA, O’Brien P (2012) iso-Propylthiobiuret-copper and indium complexes as novel precursors forcolloidal synthesis of CuInS2 nanoparticles. J Mater Chem 22:3781–3785. doi:10.1039/c2jm15460a
Akaki Y, Kurihara S, Shirahama M, Tsurugida K, Kakeno T, Yoshino K (2005) Structural and electrical characterization of AgInS2 thin films grown by single-source thermal evaporation method. J Mater Sci 16:393–396. doi:10.1007/s10854-005-2303-7
Aldana J, Wang YA, Peng X (2001) Photochemical instability of CdSe nanocrystals coated by hydrophilic thiols. J Am Chem Soc 123:8844–8850. doi:10.1021/ja016424q
Bao N, Qiu X, Wang Y-HA, Zhou Z, Lu X, Grimes CA, Gupta A (2011) Facile thermolysis synthesis of CuInS2 nanocrystals with tunable anisotropic shape and structure. Chem Commun 47:9441–9443. doi:10.1039/C1CC13314D
Batabyal SK, Tian L, Venkatram N, Ji W, Vittal JJ (2009) Phase-selective synthesis of CuInS2 nanocrystals. J Phys Chem C 113:15037–15042. doi:10.1021/jp905234y
Battaglia D, Peng X (2002) Formation of high quality InP and InAs nanocrystals in a noncoordinating solvent. Nano Lett 2(9):1027–1030. doi:10.1021/nl025687v
Castro SL, Bailey SG, Raffaelle RP, Banger KK, Hepp AF (2003) Nanocrystalline chalcopyrite materials (CuInS2 and CuInSe2) via low-temperature pyrolysis of molecular single-source precursors. Chem Mater 15:3142–3147. doi:10.1021/cm034161o
Chang J-Y, Cheng C-Y (2011) Facile one-pot synthesis of copper sulfide–metal chalcogenide anisotropic heteronanostructures in a noncoordinating solvent. Chem Commun 47:9089–9091. doi:10.1039/c1cc13150h
Chen M, Feng Y-G, Wang X, Li T-C, Zhang J-Y, Qian D-J (2007) Silver nanoparticles capped by oleylamine: FORMATION, growth, and self-organization. Langmuir 23:5296–5304. doi:10.1021/la700553d
Choi S-H, Kim E-G, Hyeon T (2006) One-pot synthesis of copper-indium sulfide nanocrystal heterostructures with acorn, bottle, and larva shapes. J Am Chem Soc 128:2520–2521. doi:10.1021/ja0577342
Dai M, Ogawa S, Kameyama T, Okazaki K, Kudo A, Kuwabata S, Tsuboi Y, Torimoto T (2012) Tunable photoluminescence from the visible to near-infrared wavelength region of non-stoichiometric AgInS2 nanoparticles. J Mater Chem 22:12851–12858. doi:10.1039/c2jm31463k
Du W, Qian X, Yin J, Gong Q (2007) Shape- and phase-controlled synthesis of monodispersed, single-crystalline ternary chalcogenide colloids through a convenient solution synthesis strategy. Chem Eur J 13:8840–8846. doi:10.1002/chem.200700618
Elim HI, Ji W, Ng MT, Vittal JJ (2007) AgInSe2 nanorods: a semiconducting material or saturable absorber. Appl Phys Lett 90:033106. doi:10.1063/1.2429030
Gaponenko SV (1998) Optical properties of semiconductor nanocrystals. Cambridge studies in modern optics. Cambridge University Press, Cambridge
Han W, Yi L, Zhao N, Tang A, Gao M, Tang Z (2008) Synthesis and shape-tailoring of copper Sulfide/indium SULfiDE-BASED NANOCRYSTALS. J Am Chem Soc 130:13152–13161. doi:10.1021/ja8046393
Huang W-C, Tseng C-H, Chang S-H, Tuan H-Y, Chiang C-C, Lyu L-M, Huang MH (2012) Solvothermal synthesis of zincblende and wurtzite CuInS2 nanocrystals and their photovoltaic application. Langmuir 28:8496–8501. doi:10.1021/la300742p
Ji X, Copenhaver D, Sichmeller C, Peng X (2008) Ligand bonding and dynamics on colloidal nanocrystals at room temperature: the case of alkylamines on CdSe nanocrystals. J Am Chem Soc 130:5726–5735. doi:10.1021/ja710909f
Joseph CM, Menon CS (1996) Electrical conductivity, optical absorption and structural studies in thin films. Semicond Sci Technol 11:1668. doi:10.1088/0268-1242/11/11/005
Lee JJ, Lee JD, Ahn BY, Kim HS, Kim KH (2007) Structural and optical properties of AgInSe2 films prepared on indium tin oxide substrates. J Korean Phys Soc 50:1099–1103. doi:10.3938/jkps.50.1099
Li L-S, Hu j, Yang W, Alivisatos AP (2001) Band gap variation of size- and shape-controlled colloidal CdSe quantum rods. Nano Lett 1(7):349–351. doi:10.1021/nl015559r
Lin L-H, Wu C-C, Lai C-H, Lee T-C (2008) Controlled deposition of silver indium sulfide ternary semiconductor thin films by chemical bath deposition. Chem Mater 20:4475–4483. doi:10.1021/cm702081h
Lu X, Zhuang Z, Peng Q, Li Y (2011) Controlled synthesis of wurtzite CuInS2 nanocrystals and their side-by-side nanorod assemblies. CrystEngComm 13:4039–4045. doi:10.1039/c0ce00451k
Nairn JJ, Shapiro PJ, Twamley B, Pounds T, von Wandruszka R, Fletcher TR, Williams M, Wang C, Norton MG (2006) Preparation of ultrafine chalcopyrite nanoparticles via the photochemical decomposition of molecular single-source precursors. Nano Lett 6:1218–1223. doi:10.1021/nl060661f
Nakamura S, Seto S (2009) Optical properties of AgInS2 thin films prepared by sulfurization of evaporated metal precursors. Phys Status Solidi C 6(5):1137–1140. doi:10.1002/pssc.200881153
Ogawa T, Kuzuya T, Hamanaka Y, Sumiyama K (2010) Synthesis of Ag–In binary sulfide nanoparticles: structural tuning and their photoluminescence properties. J Mater Chem 20:2226–2231. doi:10.1039/B920732E
Pan D, An L, Sun Z, Hou W, Yang Y, Yang Z, Lu Y (2008) Synthesis of Cu–In–S ternary nanocrystals with tunable structure and composition. J Am Chem Soc 130:5620–5621. doi:10.1021/ja711027j
Peng X, Wickham J, Alivisatos AP (1998) Kinetics of II–VI and III–V colloidal semiconductor nanocrystal growth: “Focusing” of size distributions. J Am Chem Soc 120:5343–5344. doi:10.1021/ja9805425
Peng S, Zhang S, Mhaisalkar SG, Ramakrishna S (2012) Synthesis of AgInS2 nanocrystal ink and its photoelectrical application. Phys Chem Chem Phys 14:8523–8529. doi:10.1039/c2cp40848a
Pradhan N, Reifsnyder D, Xie R, Aldana J, Peng X (2007) Surface ligand dynamics in growth of nanocrystals. J Am Chem Soc 129:9500–9509. doi:10.1021/ja0725089
Qu L, Peng X (2002) Control of photoluminescence properties of CdSe nanocrystals in growth. J Am Chem Soc 124(9):2049–2055. doi:10.1021/ja017002j
Sadtler B, Demchenko DO, Zheng H, Hughes SM, Merkle MG, Dahmen U, Wang L-W, Alivisatos AP (2009) Selective facet reactivity during cation exchange in cadmium sulfide nanorods. J Am Chem Soc 131:5285–5293. doi:10.1021/ja809854q
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst A 32:751–767. doi:10.1107/S0567739476001551
Tian L, Vittal JJ (2007) Synthesis and characterization of ternary AgInS2 nanocrystals by dual- and multiple-source methods. New J Chem 31:2083–2087. doi:10.1039/b707960e
Tian L, Elim HI, Ji W, Vittal JJ (2006) One-pot synthesis and third-order nonlinear optical properties of AgInS2 nanocrystals. Chem Commun 4276–4278. doi:10.1039/b607855a
Venkatram N, Batabyal SK, Tian L, Vittal JJ, Ji W (2009) Shape-dependent nonlinear absorption and relaxation in CuInS2 nanocrystals. Appl Phys Lett 95:201109. doi:10.1063/1.3266518
Vittal JJ, Ng MT (2006) Chemistry of metal thio- and selenocarboxylates: precursors for metal sulfide/selenide materials, thin films, and nanocrystals. Acc Chem Res 39:869–877. doi:10.1021/ar050224s
Wang D, Zheng W, Hao C, Peng Q, Li Y (2008) General synthesis of I–III–VI2 ternary semiconductor nanocrystals. Chem Commun 2556–2558. doi:10.1039/b800726h
Xie R, Rutherford M, Peng X (2009) Formation of high-quality I–III–VI semiconductor nanocrystals by tuning relative reactivity of cationic precursors. J Am Chem Soc 131:5691–5697. doi:10.1021/ja9005767
Yin Y, Alivisatos P (2005) Colloidal nanocrystal synthesis and the organic–inorganic interface. Nature 437:7059. doi:10.1038/nature04165
Yue W, Han S, Peng R, Shen W, Geng H, Wu F, Tao S, Wang M (2010) CuInS2 quantum dots synthesized by a solvothermal route and their application as effective electron acceptors for hybrid solar cells. J Mater Chem 20:7570–7578. doi:10.1039/c0jm00611d
Zhong H, Li Y, Ye M, Zhu Z, Zhou Y, Yang C, Li Y (2007) A facile route to synthesize chalcopyrite CuInSe2 nanocrystals in non-coordinating solvent. Nanotechnology 18:025602. doi:10.1088/0957-4484/18/2/025602
Acknowledgments
Financial support for this study was granted by the Ministry of Education and Science of the Republic of Serbia (Projects: III45020 and OI172056). This research was also supported by COST Actions CM1101 and MP1106.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Beloš, M.V., Abazović, N.D., Jakovljević, J.K. et al. Influence of sulphide precursor on crystal phase of ternary I–III–VI2 semiconductors. J Nanopart Res 15, 2148 (2013). https://doi.org/10.1007/s11051-013-2148-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-013-2148-6