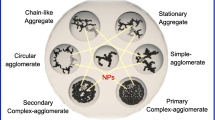
Abstract
The estimation of nanoparticle agglomerates’ size in fluidized beds remains an open challenge, mainly due to the difficulty of characterizing the inter-agglomerate van der Waals force. The current approach is to describe micron-sized nanoparticle agglomerates as micron-sized particles with 0.1–0.2-μm asperities. This simplification does not capture the influence of the particle size on the van der Waals attraction between agglomerates. In this paper, we propose a new description where the agglomerates are micron-sized particles with nanoparticles on the surface, acting as asperities. As opposed to previous models, here the van der Waals force between agglomerates decreases with an increase in the particle size. We have also included an additional force due to the hydrogen bond formation between the surfaces of hydrophilic and dry nanoparticles. The average size of the fluidized agglomerates has been estimated equating the attractive force obtained from this method to the weight of the individual agglomerates. The results have been compared to 54 experimental values, most of them collected from the literature. Our model approximates without a systematic error the size of most of the nanopowders, both in conventional and centrifugal fluidized beds, outperforming current models. Although simple, the model is able to capture the influence of the nanoparticle size, particle density, and Hamaker coefficient on the inter-agglomerate forces.



Similar content being viewed by others
References
Ackler HD, French RH, Chiang YM (1996) Comparisons of hamaker constants for ceramic systems with intervening vacuum or water: from force laws and physical properties. J Colloid Interface Sci 179(2):460–469
Castellanos A (2005) The relationship between attractive interparticle forces and bulk behaviour in dry and uncharged fine powders. Adv Phys 54(4):263–376
Castellanos A, Valverde JM, Quintanilla MAS (2001) Aggregation and sedimentation in gas-fluidized beds of cohesive powders. Phys Rev E 64:041,304
Chaouki J, Chavarie C, Klvana D, Pajonk G (1985) Effect of interparticle forces on the hydrodynamic behaviour of fluidized aerogels. Powder Technol 43(2):117–125
de Martín L, Bouwman WG, van Ommen JR (2012) Two-level hierarchical structure in nano-powder agglomerates in gas media. In: Bulleting of the Americal Physical Society, vol 57
Espin MJ, Valverde JM, Quintanilla MAS, Castellanos A (2009) Electromechanics of fluidized beds of nanoparticles. Phys Rev E 79:011,304
Forsyth AJ, Rhodes MJ (2000) A simple model incorporating the effects of deformation and asperities into the van der waals force for macroscopic spherical solid particles. J Colloid Interface Sci 223(1):133–138
French RH, Cannon RM, DeNoyer LK, Chiang YM (1994) Full spectral calculation of non-retarded hamaker constants for ceramic systems from interband transition strengths. Solid State Ion 75:13–33
Friedlander SK (2000) Smoke, dust, and haze: fundamentals of aerosol dynamics, 2nd edn. Oxford University Press, USA
Israelachvili JN (2011) Intermolecular and surface forces, 3rd edn. Academic Press, London
Katainen J, Paajanen M, Ahtola E, Pore V, Lahtinen J (2006) Adhesion as an interplay between particle size and surface roughness. J Colloid Interface Sci 304(2):524–529
Kim HY, Sofo JO, Velegol D, Cole MW, Lucas AA (2007) Van der waals dispersion forces between dielectric nanoclusters. Langmuir 23(4):1735–1740
Krupp H (1967) Particle adhesion, theory and experiment. Adv Colloid Interface Sci 1:111–239
Li Q, Rudolph V, Peukert W (2006) London-van der waals adhesiveness of rough particles. Powder Technol 161(3):248–255
Matsuda S, Hatano H, Tsutsumi A (2001) Ultrafine particle fluidization and its application to photocatalytic NOx treatment. Chem Eng J 82(13):183–188
Matsuda S, Hatano H, Muramoto T, Tsutsumi A (2004) Modeling for size reduction of agglomerates in nanoparticle fluidization. AIChE J 50(11):2763–2771
Nam CH, Pfeffer R, Dave RN, Sundaresan S (2004) Aerated vibrofluidization of silica nanoparticles. AIChE J 50(8):1776–1785
Quintanilla MAS, Valverde JM, Espin MJ, Castellanos A (2012) Electrofluidization of silica nanoparticle agglomerates. Ind Eng Chem Res 51(1):531–538
Rabinovich YI, Adler JJ, Ata A, Singh RK, Moudgil BM (2000) Adhesion between nanoscale rough surfaces: I. Role of asperity geometry. J Colloid Interface Sci 232(1):10–16
Rumpf H (1990) Particle technology. Chapman & Hall, London
Shabanian J, Jafari R, Chaouki J (2012) Fluidization of ultrafine powders. Int Rev Chem Eng 4(1):16–50
Tahmasebpoor M, de Martín L, Talebi M, Mostoufi N, van Ommen JR (2013) The role of the hydrogen bond in dense nanoparticle-gas suspensions. Phys Chem Chem Phys 15:5788–5793
Valverde JM, Castellanos A (2006) Fluidization of nanoparticles: a modified Richardson–Zaki law. AIChE J 52(2):838–842
Valverde JM, Castellanos A (2008a) Fluidization of nanoparticles: A simple equation for estimating the size of agglomerates. Chem Eng J 140(13):296–304
Valverde JM, Castellanos A (2008b) A modified Richardson–Zaki equation for fluidization of geldart B magnetic particles. Powder Technol 181(3):347–350
van Ommen JR, Valverde JM, Pfeffer R (2012) Fluidization of nanopowders: a review. J Nanopart Res 14(3):737–766
Wang XS, Palero V, Soria J, Rhodes MJ (2006a) Laser-based planar imaging of nano-particle fluidization: Part I. Determination of aggregate size and shape. Chem Eng Sci 61(16):5476–5486
Wang XS, Palero V, Soria J, Rhodes MJ (2006b) Laser-based planar imaging of nano-particle fluidization: Part II. Mechanistic analysis of nanoparticle aggregation. Chem Eng Sci 61(24):8040–8049
Wu MK, Friedlander SK (1993) Note on the power law equation for fractal-like aerosol agglomerates. J Colloid Interface Sci 159(1):246–248
Yao W, Guangsheng G, Fei W, Jun W (2002) Fluidization and agglomerate structure of SiO2 nanoparticles. Powder Technol 124(12):152–159
Zhou L, Zhang F, Zhou T, Kage H, Mawatari Y (2013) A model for estimating agglomerate sizes of non-magnetic nanoparticles in magnetic fluidized beds. Korean J Chem Eng 30(2):501–507
Acknowledgments
The research leading to these results has received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP/ 2007–2013) / ERC Grant, Agreement no 279632.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Martín, L., van Ommen, J.R. A model to estimate the size of nanoparticle agglomerates in gas−solid fluidized beds. J Nanopart Res 15, 2055 (2013). https://doi.org/10.1007/s11051-013-2055-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-013-2055-x