Abstract
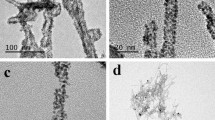
Peritoneal metastases of colorectal cancer are a significant challenge in the field of medicine today due to poor results of systemic chemotherapy caused by the poor diffusion of drugs across the blood–peritoneal barrier. Multi-walled carbon nanotubes (MWNTs) are a biocompatible nanomaterial that strongly absorb near-infrared light to locally heat the surrounding area. Colorectal cancer is known to overexpress folate receptor; therefore, folic acid (FA) was covalently attached to MWNTs to target colorectal cancer cells. Results from real-time polymerase chain reaction found differing expression of folate receptor-α in two colorectal cancer cell lines, RKO and HCT116, as well as a healthy epithelial cell line, HEPM. A spectrophotometric method was developed to quantify the mass of MWNTs bound to cells, and it was determined that FA-targeted MWNTs resulted in a 400–500 % greater affinity for colorectal cancer cells than untargeted MWNTs. The non-cancerous cell line, HEPM, had higher non-specific MWNT interaction and similar MWNT–FA affinity. Stimulated by 1,064 nm light, FA-functionalized MWNTs caused a 50–60 % decrease in colorectal cancer cell viability compared to a 4–10 % decrease caused by untargeted MWNTs. Our results indicate that FA-targeted MWNTs may increase the therapeutic index of MWNT-induced photothermal therapy.









Similar content being viewed by others
References
Ali-Boucetta H, Al-Jamal KT, McCarthy D, Prato M, Bianco A, Kostarelos K (2008) Multiwalled carbon nanotube-doxorubicin supramolecular complexes for cancer therapeutics. Chem Commun 4:459–461
American Cancer Society. Cancer Facts and Figures 2011
Bahr JL, Tour JM (2002) Covalent chemistry of single-wall carbon nanotubes. J Mater Chem 12(7):1952–1958. doi:10.1039/B201013p
Bianco A, Prato M (2003) Can carbon nanotubes be considered useful tools for biological applications? Adv Mater 15(20):1765–1768
Bianco A, Kostarelos K, Prato M (2005) Applications of carbon nanotubes in drug delivery. Curr Opin Chem Biol 9(6):674–679. doi:10.1016/j.cbpa.2005.10.005
Burke A, Ding XF, Singh R, Kraft RA, Levi-Polyachenko N, Rylander MN, Szot C, Buchanan C, Whitney J, Fisher J, Hatcher HC, D’Agostino R, Kock ND, Ajayan PM, Carroll DL, Akman S, Torti FM, Torti SV (2009) Long-term survival following a single treatment of kidney tumors with multiwalled carbon nanotubes and near-infrared radiation. Proc Natl Acad Sci USA 106(31):12897–12902
Dhar S, Liu Z, Thomale J, Dai HJ, Lippard SJ (2008) Targeted single-wall carbon nanotube-mediated Pt(IV) prodrug delivery using folate as a homing device. J Am Chem Soc 130(34):11467–11476
Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, Felix R, Riess H (2002) The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol 43(1):33–56
Huang H, Yuan Q, Shah JS, Misra RD (2011a) A new family of folate-decorated and carbon nanotube-mediated drug delivery system: synthesis and drug delivery response. Adv Drug Deliv Rev 63(14–15):1332–1339. doi:10.1016/j.addr.2011.04.001
Huang H, Yuan Q, Shah JS, Misra RDK (2011b) A new family of folate-decorated and carbon nanotube-mediated drug delivery system: synthesis and drug delivery response. Adv Drug Deliv Rev 63(14–15):1332–1339
Kam NWS, O’Connell M, Wisdom JA, Dai HJ (2005) Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci USA 102(33):11600–11605
Levi-Polyachenko NH, Merkel EJ, Jones BT, Carroll DL, Stewart JH (2009) Rapid photothermal intracellular drug delivery using multiwalled carbon nanotubes. Mol Pharmaceut 6(4):1092–1099
Li RB, Wu R, Zhao L, Wu MH, Yang L, Zou HF (2010) P-Glycoprotein antibody functionalized carbon nanotube overcomes the multidrug resistance of human leukemia cells. ACS Nano 4(3):1399–1408
Liu Z, Sun XM, Nakayama-Ratchford N, Dai HJ (2007) Supramolecular chemistry on water-soluble carbon nanotubes for drug loading and delivery. ACS Nano 1(1):50–56
Liu Z, Chen K, Davis C, Sherlock S, Cao QZ, Chen XY, Dai HJ (2008) Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res 68(16):6652–6660
Liu ZA, Yang K, Lee ST (2011) Single-walled carbon nanotubes in biomedical imaging. J Mater Chem 21(3):586–598
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25(4):402–408. doi:10.1006/meth.2001.1262
Marches R, Chakravarty P, Musselman IH, Bajaj P, Azad RN, Pantano P, Draper RK, Vitetta ES (2009) Specific thermal ablation of tumor cells using single-walled carbon nanotubes targeted by covalently-coupled monoclonal antibodies. Int J Cancer 125(12):2970–2977
Mittal JP (1995) Excited-states and electron-transfer reactions of fullerenes. Pure Appl Chem 67(1):103–110
Mohapatra S, Mallick SK, Maiti TK, Ghosh SK, Pramanik P (2007) Synthesis of highly stable folic acid conjugated magnetite nanoparticles for targeting cancer cells. Nanotechnology 18(38):385102
Moon HK, Lee SH, Choi HC (2009) In vivo near-infrared mediated tumor destruction by photothermal effect of carbon nanotubes. ACS Nano 3(11):3707–3713
Osswald S, Flahaut E, Ye H, Gogotsi Y (2005) Elimination of D-band in Raman spectra of double-wall carbon nanotubes by oxidation. Chem Phys Lett 402(4–6):422–427
Rosca ID, Watari F, Uo M, Akaska T (2005) Oxidation of multiwalled carbon nanotubes by nitric acid. Carbon 43(15):3124–3131. doi:10.1016/j.carbon.2005.06.019
Ross JF, Chaudhuri PK, Ratnam M (1994) Differential regulation of folate receptor isoforms in normal and malignant-tissues in-vivo and in established cell-lines—physiological and clinical implications. Cancer 73(9):2432–2443
Sideratou Z, Tsiourvas D, Theodossiou T, Fardis M, Paleos CM (2010) Synthesis and characterization of multifunctional hyperbranched polyesters as prospective contrast agents for targeted MRI. Bioorg Med Chem Lett 20(14):4177–4181
Torti SV, Byrne F, Whelan O, Levi N, Ucer B, Schmid M, Torti FM, Akman S, Liu J, Ajayan PM, Nalamasu O, Carroll DL (2007) Thermal ablation therapeutics based on CN(x) multi-walled nanotubes. Int J Nanomedicine 2(4):707–714
Velasco-Santos C, Martinez-Hernandez AL, Lozada-Cassou M, Alvarez-Castillo A, Castano VM (2002) Chemical functionalization of carbon nanotubes through an organosilane. Nanotechnology 13(4):495–498
Vortherms AR, Doyle RP, Gao D, Debrah O, Sinko PJ (2008) Synthesis, characterization, and in vitro assay of folic acid conjugates of 3′-azido-3′-deoxythymidine (AZT): toward targeted AZT based anticancer therapeutics. Nucleosides Nucleotides Nucleic Acids 27(2):173–185. doi:10.1080/15257770701795946
Wang S, Low PS (1998) Folate-mediated targeting of antineoplastic drags, imaging agents, and nucleic acids to cancer cells. J Control Release 53(1–3):39–48
Yu SS, Lau CM, Thomas SN, Jerome WG, Maron DJ, Dickerson JH, Hubbell JA, Giorgio TD (2012) Size- and charge-dependent non-specific uptake of PEGylated nanoparticles by macrophages. Int J Nanomed 7:799–813. doi:10.2147/IJN.S28531ijn-7-799
Yuan JS, Reed A, Chen F, Stewart CN Jr (2006) Statistical analysis of real-time PCR data. BMC Bioinform 7:85. doi:10.1186/1471-2105-7-85
Zhang X, Meng L, Lu Q, Fei Z, Dyson PJ (2009) Targeted delivery and controlled release of doxorubicin to cancer cells using modified single wall carbon nanotubes. Biomaterials 30(30):6041–6047. doi:10.1016/j.biomaterials.2009.07.025
Zhu J, Kim JD, Peng HQ, Margrave JL, Khabashesku VN, Barrera EV (2003) Improving the dispersion and integration of single-walled carbon nanotubes in epoxy composites through functionalization. Nano Lett 3(8):1107–1113. doi:10.1021/Nl0342489
Acknowledgments
We thank the Department of Plastic and Reconstructive Surgery at Wake Forest University Baptist Medical Center for funding and the Department of Chemistry at Wake Forest University for the use of the Raman, ESI–MS, NMR, and FTIR.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Graham, E.G., MacNeill, C.M. & Levi-Polyachenko, N.H. Quantifying folic acid-functionalized multi-walled carbon nanotubes bound to colorectal cancer cells for improved photothermal ablation . J Nanopart Res 15, 1649 (2013). https://doi.org/10.1007/s11051-013-1649-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-013-1649-7