Abstract
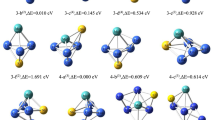
The growth behavior of Co n Al (n = 1–15) and the chemisorptions of hydrogen on the ground state geometries have been studied using the density functional theory (DFT) within the generalized gradient approximation (GGA). The growth pattern for Co n Al is Al-substituted Co n+1 clusters, and it keeps the similar frameworks of the most stable Co n+1 clusters except for n = 2, 3, and 6. The Al atom substitutes the surface atom of the Co n+1 clusters for n ≤ 13. Starting from n = 14, the Al atom completely falls into the center of the Co-frame. The dissociation energy, the second-order energy differences, and the HOMO–LUMO gaps indicate that the magic numbers of the calculated Co n Al clusters are 7, 9, and 13, corresponding to the high symmetrical structures. To my knowledge, this is the first time that a systematic study of chemisorption of hydrogen on cobalt aluminum clusters. The twofold bridge site is identified to be the most favorable chemisorptions site for one hydrogen adsorption on Co n Al (n = 1–6, 8, 10), and two hydrogen adsorption on Co n Al (n = 1–7), while threefold hollow site is preferred for one hydrogen adsorption on Co n Al (n = 7, 9, 11–15) and two hydrogen adsorption on Co n Al (n = 8–10, 12–15) clusters. The ground state structure of two hydrogen adsorption on Co11Al is exceptional. In general, the binding energy of both H and 2H of Co n Al (n = 1–12) is found to increase with the cluster size. And the result shows that large binding energies of the hydrogen atoms and large fragmentation energies for Co11AlH and Co12AlH make these species behaving like magic clusters.







Similar content being viewed by others
References
Behm JM, Brugh DJ, Morse MD (1994) Spectroscopic analysis of the open 3d subshell transition metal aluminides: AlV, AlCr, and AlCo. J Chem Phys 101(8):6487–6499
Delley B (1990) An all-electron numerical method for solving the local density functional for polyatomic molecules. J Chem Phys 92(1):508–517
Dhilip Kumar TJ, Tarakeshwar P, Balakrishnan N (2009) Geometric and electronic structures of hydrogenated transition metal (Sc, Ti, Zr) clusters. Phys Rev B 79(20): 205415-1-11
Hales DA, Su CX, Lian L, Armentrout BP (1994) Collisioninduced dissociation of Co + n (n = 2–18) with Xe: bond energies of cationic and neutral cobalt clusters, dissociation pathways, and structures. J Chem Phys 100(2):1049–1057
Huda MN, Kleinman L (2006) Hydrogen adsorption and dissociation on small platinum clusters: an electronic structure density functional study. Phys Rev B 74(19):195407-1-7
Kant A, Strauss B (1964) Dissociation energies of diatomic molecules of the transition elements. II. Titanium, chromium, manganese, and cobalt. J Chem Phys 41(12):3806–3808
Laguna A, Lasanta T, Lopez-de-Luzuriaga JM, Monge M, Naumov P, Olmos ME (2010) Combining aurophilic interactions and halogen bonding to control the luminescence from bimetallic gold silver clusters. J Am Chem Soc 132(2):456–457
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37(2):785–789
Lu C, Kuang XY, Lu ZW, Mao AJ, Ma YM (2011) Determination of structures, stabilities, and electronic properties for bimetallic cesium-doped gold clusters: a density functional theory study. J Phys Chem A 115(33):9273–9281
Ma QM, Xie Z, Wang J, Liu Y, Li YC (2006) Structures, stabilities and magnetic properties of small Co clusters. Phys Lett A 358(4):289–296
Menezes WJC, Knickelbein MB (1991) Bimetallic clusters of cobalt and aluminum: ionization potentials versus reactivity, and the importance of geometric structure. Chem Phys Lett 183(5–6):357–362
Menezes WJC, Knickelbein MB (1993) The evolution of electronic structure in Al n Co m . Z Phys D 26:322–325
Nonose S, Sone Y, Onodera K, Sudo S, Kaya K (1989) Reactivity study of alloy clusters made of aluminum and some transition metals with hydrogen. Chem Phys Lett 164(4):427–432
Pramann A, Nakajima A, Kaya K (2001) Photoelectron spectroscopy of bimetallic aluminum cobalt cluster anions: comparison of electronic structure and hydrogen chemisorption rates. J Chem Phys 115(12):5404–5410
Rosen B (1970) Spectroscopic data relative to diatomic molecules. Pergamon, Oxford
Varano A, Henry DJ, Yarovsky I (2010) DFT study of H adsorption on magnesium-doped aluminum clusters. J Phys Chem A 114(10):3602–3608
Wang SY, Yu JZ, Mizuseki H, Yan JA (2004) First-principles study of the electronic structures of icosahedral TiN(N = 13,19,43,55) clusters. J Chem Phys 120(18):8463–8468
Xie Z, Ma QM, Liu Y, Li YC (2005) First-principles study of the stability and Jahn-Teller distortion of nickel clusters. Phys Lett A 342(5):459–467
Zanti G, Peeters D (2010) DFT study of bimetallic palladium gold clusters Pd n Au m of low nuclearities (n + m <14). J Phys Chem A 114(38):10345–10356
Zhang DB, Shen J (2004) Ground state, growth, and electronic properties of small lanthanum clusters. J Chem Phys 120(11):5104–5109
Zhao YR, Kuang XY, Zheng BB, Li YF, Wang SJ (2011) Equilibrium geometries, stabilities, and electronic properties of the bimetallic M2-doped Au n (M = Ag, Cu; n = 1–10) clusters: comparison with Pure Gold clusters. J Phys Chem A 115(5):569–576
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant No. 20603021), Youth Foundation of Shanxi (Grant No. 2007021009), and the Youth Academic Leader of Shanxi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, L. Evolution of Co n Al clusters and chemisorption of hydrogen on Co n Al clusters. J Nanopart Res 14, 957 (2012). https://doi.org/10.1007/s11051-012-0957-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-012-0957-7