Abstract
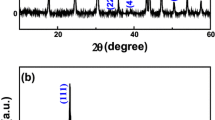
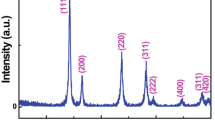
CeO2 hollow nanospheres were synthesized by a low-cost and environmentally benign one-pot hydrothermal route. Templates, surfactants, or other auxiliaries were not used in the route. X-ray diffraction, transmission electron microscopy, scanning electron microscopy, X-ray photoelectron spectroscopy, high-resolution transmission electron microscopy, and nitrogen adsorption–desorption measurements were used to characterize the products. The average diameter of hollow spheres, with shells of approximately 30 nm, was about 300 nm. The formation of these hollow spheres involved a transformation from Ce(OH)CO3 solid spheres to CeO2 hollow nanospheres. The CeO2 hollow nanospheres exhibited a higher catalytic activity on CO oxidation than CeO2 nano-octahedrons.









Similar content being viewed by others
References
Barreca D, Comini E, Gasparotto A, Maccato C, Maragno C, Sberveglieri G et al (2008) Gas sensing properties of columnar CeO2 nanostructures prepared by chemical vapor deposition. J Nanosci Nanotechnol 8:1012–1016
Bêche E, Charvin P, Perarnau D, Abanades S, Flamant G (2008) Ce 3d XPS investigation of cerium oxides and mixed cerium oxide (cextiyoz). Surf Interface Anal 40:264–267
Campbell CT, Peden CHF (2005) Chemistry—oxygen vacancies and catalysis on ceria surfaces. Science 309:713–714
Cao CY, Cui Z, Chen CQ, Song WG, Cai W (2010) Ceria hollow nanospheres produced by a template-free microwave-assisted hydrothermal method for heavy metal ion removal and catalysis. J Phys Chem C 114:9865–9870
Chen GZ, Sun SX, Sun X, Fan WL, You T (2009) Formation of CeO2 nanotubes from Ce(OH)CO3 nanorods through Kirkendall diffusion. Inorg Chem 48:1334–1338
Chen S, Yu SH, Yu B, Ren L, Yao W, Cölfen H (2004) Solvent effect on mineral modification: selective synthesis of cerium compounds by a facile solution route. Chem Eur J 10:3050–3058
Cheng DG, Chong MB, Chen FQ, Zhan XL (2008) XPS characterization of CeO2 catalyst for hydrogenation of benzoic acid to benzaldehyde. Catal Lett 120:82–85
Dudek M (2008) Ceramic oxide electrolytes based on CeO2-preparation, properties and possibility of application to electrochemical devices. J Eur Ceram Soc 28:965–971
Guo ZT, Du FL, Li GC, Cui ZL (2006) Synthesis and characterization of single-crystal Ce(OH)CO3 and CeO2 triangular microplates. Inorg Chem 45:4167–4169
Huang PX, Wu F, Zhu BL, Gao XP, Zhu HY, Yan TY et al (2005) CeO2 nanorods and gold nanocrystals supported on CeO2 nanorods as catalyst. J Phys Chem B 109:19169–19174
Natile MM, Glisenti A (2005) CoOx/ CeO2 nanocomposite powders: synthesis, characterization, and reactivity. Chem Mater 17:3403–3414
Oh MH, Nho JS, Cho SB, Lee JS, Singh RK (2011) Polishing behaviors of ceria abrasives on silicon dioxide and silicon nitride cmp. Powder Technol 206:239–245
Phokha S, Maensiri S (2010) Hydrothermal synthesis and characterization of monodisperse CeO2 nanospheres, vols 1 and 2. Inec: 2010 3rd international nanoelectronics conference, Hongkong, pp 545–546
Shen GL, Wang Q, Wang Z, Chen YF (2011) Hydrothermal synthesis of CeO2 nano-octahedrons. Mater Lett 65:1211–1214
Strandwitz NC, Stucky GD (2009) Hollow microporous cerium oxide spheres templated by colloidal silica. Chem Mater 21:4577–4582
Wu GS, Xie T, Yuan XY, Cheng BC, Zhan LD (2004) An improved sol-gel template synthetic route to large-scale CeO2 nanowires. Mater Res Bull 39:1023–1028
Yan L, Yu RB, Chen J, Xing XR (2008) Template-free hydrothermal synthesis of CeO2 nano-octahedrons and nanorods: Investigation of the morphology evolution. Cryst Growth Des 8:1474–1477
Yang ZJ, Han DQ, Ma DL, Liang H, Liu L, Yang YZ (2010) Fabrication of monodisperse CeO2 hollow spheres assembled by nano-octahedra. Cryst Growth Des 10:291–295
Acknowledgments
This work was supported financially by the National Natural Science Foundation of China (No. 90406024) and the National 863 Program (No. 2007AA061401).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, G., Liu, H., Wang, Q. et al. Self-template hydrothermal synthesis of CeO2 hollow nanospheres. J Nanopart Res 14, 954 (2012). https://doi.org/10.1007/s11051-012-0954-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-012-0954-x