Abstract
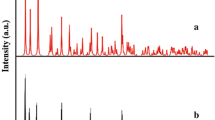
A simple solvothermal method for the selective synthesis of β-HgS (meta cinnabar) nanoparticles in aqueous solutions is reported with bis(dibenzyldithiocarbamato)mercury(II) as the precursor. Crystal structure, size, morphology and composition of the products are characterized by X-ray powder diffraction (XRD), scanning electron microscopy (SEM), energy-dispersive X-ray (EDX) analysis, high-resolution transmission electron microscopy (HRTEM), SAED and X-ray photoelectron spectroscopy (XPS). PXRD shows (111), (220), (200), (311), (222), (400), (331), (420) reflections characteristic of β-HgS. SEM micrographs display the spherical nature of the nano-β-HgS. EDX analysis showed the presence of Hg and S. HRTEM images indicate the spherical nature of the nanoparticles with their size in the range of 10–15 nm and the FFT pattern shows the crystalline nature of the spherical particles. The results are in agreement with those estimated from the XRD pattern. XPS signals observed at 162.6 and 162.8 eV are due to S2p 3/2 and S2p 1/2 electrons and the S2s was observed at 222.3 eV. The band gap of nano-β-HgS has been found to be 3.6 eV from the UV–visible spectral measurement. The blue-shifted band gap compared to the bulk HgS is a consequence of “size quantization” effect. A comprehensive characterization of the precursor by IR and single crystal X-ray crystallography shows the presence of HgS4 coordination environment, with a distinct Hg–S bond asymmetry.










Similar content being viewed by others
References
Afzaal M, Ellwood K, Pickeft NL, Brien PO’, Raftery J, Walfers J (2004) Growth of lead chalcogenide thin films using single-source precursors. J Mater Chem 14:1310–1315. doi:10.1039/B313063K
Alivisatos P (2004) The use of nanocrystals in biological detection. Nat Biotechnol 22:47–52. doi:10.1038/nbt927
ArulPrakasam B, Ramalingam K, Bocelli G, Cantoni A (2009) Spectral, BVS, and thermal studies on bisdithiocarbamates of divalent Zn, Cd, and their adducts: Single crystal X-ray structure redetermination of (diiodo) (tetraethylthiuramdisulfide)mercury(II), [Hg(tetds)I2]. Phosphor Sulfur Silicon Relat Elem 184:2020–2033. doi:10.1080/10426500802418016
Brese NE, Keeffe MO (1991) Bond-valence parameters for solids. Acta Crystallogr B47:192–197. doi:10.1107/S0108768190011041
Bruker, SADABS (version 2008, 1-0). Bruker AXS Inc., Madison, Wisconsin, USA
Casas JS, Sanchez A, Bravo J, Garcia-Fontan S, Castellano EE, Jones MM (1989) Cadmium coordination chemistry related to chelate therapy. Inorg Chim Acta 158:119–126. doi:10.1016/S0020-1693(00)84021-9
Chieh C, Leung LPC (1976) Crystal structure and vibrational spectra of methyl, diethyldithiocarbamatomercury(II). Can J Chem 54:3077–3084. doi:10.1139/v76-438
Dai H, Wang EW, Lu YZ, Fan SS, Lieber CM (1995) Synthesis and characterization of carbide nano rods. Nature 375:769–772. doi:10.1038/375769a0
Delin A, Kluner T (2002) Excitation spectra and ground-state properties from density-functional theory for the inverted band-structure systems β-HgS, HgSe, and HgTe. Phys Rev B 66:035117. doi:10.1103/PhysRevB.66.035117
Faruggia LJ (1999) ORTEP-3 for Windows. University of Glasgow, Scotland
Fuhrer MS, Nygard J, Shih L, Forero M, Yoon YG, Mazzoni MSC, Choi HJ (2000) Crossed nanotube junctions. Science 288:494–497. doi:10.1126/science.288.5465.494
Green M, Prince P, Gardener M, Steed J (2004) Mercury(II)N,N-methyl-phenyl ethyldithiocarbamate and its use as a precursor for the room-temperature solution deposition of β-HgS thin films. Adv Mater 12:994–996. doi:10.1103/PhysRevB.66.035117
Higginson KA, Kuno M, Bonevich J, Qadri SB, Yousuf M, Mattoussi H (2002) Synthesis and characterization of colloidal β-HgS quantum dots. J Phys Chem B 106:9982–9985. doi:10.1021/jp026232x
Jones MM, Jones SG (1983) Structure–activity relationship with therapeutic chelating agents. Inorg Chim Acta 79:288–289. doi:10.1016/S0020-1693(00)95336-2
Kale SS, Lokhande CD (1999) Preparation and characterization of HgS films by chemical deposition. Mater Chem Phys 59:242–246. doi:10.1016/S0254-0584(99)00048-6
Keeffe MO (1989) Struct Bond (Berlin) 71:162–190. doi:10.1007/3-540-50775-2_5
Keeffe MO, Brese NE (1991) Atom sizes and bond lengths in molecules and crystals. J Am Chem Soc 113:3226–3229. doi:10.1021/ja00009a002
Kim S, Lim YT, Soltesz EG, Grand AMD, Lee J, Nakayama A, Parker T, Mihaljevic JA, Laurence RG, Dor DM, Cohn LH, Bawendi MG (2004) Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat Biotechnol 22:93–97. doi:10.1038/nbt920
Klug H, Alexander L (eds) (1962) X-ray diffraction procedures. Wiley, New York, pp 125–140
Kuno M, Higginson KA, Qadri SB, Yousuf M, Lee SH, Davis BL, Mattoussi H (2003) Molecular clusters of binary and ternary mercury chalcogenides: colloidal synthesis, characterization, and optical spectra. J Phys Chem B 107:5758–5767. doi:10.1002/chin.200336009
Lai CS, Tiekink ERT (2002) Phenyl(pyrrolinedithiocarbamato)mercury(II). Acta Crystallogr E 58:674–675. doi:10.1107/S1600536802019372
Lai CS, Tiekink ERT (2003) Supramolecular association in organomercury(II) 1,1-dithiolates. Complementarity between Hg-S and hydrogen bonding interactions in organomercury(II) 2-amino-cyclopent-1-ene-1-carbodithioates. Cryst Eng Com 5:253–261. doi:10.1039/B305363F
Lakshmikumar ST, Rastogi AC (1994) Selenization of Cu and In thin films for the preparation of selenide photo-absorber layers in solar cells using Se vapour source. Sol Energy Mater Sol Cells 32:7–19. doi:10.1016/0927-0248(94)90251-8
Lan GY, Lin YW, Lin ZH, Chang HT (2010) Synthesis and characterization of ZnxHg1 − xSeyS1 − y quantum dots. J Nanopart Res 12:1377–1388. doi:10.1007/s11051-009-9683-1
Li H, Zhu Y, Chen S, Palchik O, Xiong J, Koltypin Y, Gofer AG (2003) A novel ultrasound-assisted approach to the synthesis of CdSe and CdS nanoparticles. J Solid State Chem 172:102–110. doi:10.1016/S0022-4596(02)00138-X
Mahapatra AK, Dash K (2006) Synthesis and characterization of colloidal β-HgS quantum dots. Physica E 35:9–15. doi:10.1016/j.physe.2006.03.164
Marimuthu G, Ramalingam K, Rizzoli C (2010) Synthesis, spectral and thermal studies of 2,2′-bipyridyl adducts of bis(N-alkyl-N-phenyldithiocarbamato)zinc(II). Polyhedron 29:1555–1560. doi:10.1016/j.poly.2010.02.001
Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S (2005) Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307:538–544. doi:10.1126/science.1104274
Mongellaz F, Fillot A, Griot R, De Lalle LG (1994) Thermoelectric cooler for infrared detectors. Proc SPIE Int Soc Opt Eng 156:12–17. doi:10.1117/12.178600
Nieuwenhuizen J, Ehless AW, Haaghoot JG, Janse SR, Reedijk J, Baerends J (1999) The mechanism of zinc(II)-dithiocarbamate accelerated vulcanization uncovered; Theoretical and experimental evidence. J Am Chem Soc 121:163–168. doi:10.1021/ja982217n
Ramalingam K, Uma S, Rizzoli C, Marimuthu G (2010) Supramolecular interactions in high molecular weight bisdithiocarbamate adducts of divalent Zn(II), Cd(II) and Hg(II): spectral, VBS, and single crystal X-ray structural studies on MS4N2 chromophores. J Coord Chem 63:4123–4135. doi:10.1080/00958972.2010.528409
Sreekumari Nair P, Radhakrishnan T, Revaprasadu N, Kolawole GA, Brien PO (2004) The synthesis of HgS nanoparticles in polystyrene matrix. J Mater Chem 14:581–584. doi:10.1039/B304098B
Thorp HH (1992) Bond valence sum analysis of metal-ligand bond lengths in metalloenzymes and model complexes. Inorg Chem 31:1585–1588. doi:10.1021/ic00035a012
Tokyo N (1975) Optical absorption and dispersion in rf-sputtered α-HgS films. J Appl Phys 46:4857–4862. doi:10.1063/1.321519
Tsunekawa S, Fukuda T, Kasuya A (2000) Blue shift in ultraviolet absorption spectra of monodisperse CeO2-nanoparticles. J Appl Phys 87:1318–1321. doi:10.1063/1.372016
Wang H, Zhang JR, Zhu JJA (2001) Microwave assisted heating method for the rapid synthesis of sphalrite-type mercury sulfide nanocrystals with different sizes. J Cryst Growth 233:829–836. doi:10.1016/S0022-0248(01)01629-3
Wentian L, Thorp HH (1993) Bond valence sum analysis of metal-ligand bond lengths in metalloenzymes and model complexes. 2. Refined distances and other enzymes. Inorg Chem 32:4102–4105. doi:10.1021/ic00071a023
Zhu JJ, Liu SW, Chen S, Palchik O, Koltypin Y, Gedanken A (2000) A novel sonochemical method for the preparation of nanophasic sulfides: Synthesis of HgS and PbS nanoparticles. J Solid State Chem 153:342–348. doi:10.1006/jssc.2000.8780
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marimuthu, G., Ramalingam, K., Rizzoli, C. et al. Solvothermal preparation of nano-β-HgS from a precursor, bis(dibenzyldithiocarbamato)mercury(II). J Nanopart Res 14, 710 (2012). https://doi.org/10.1007/s11051-011-0710-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-011-0710-7