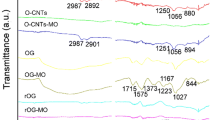
Abstract
Nanodiamond (ND) and other nanocarbon particles are popular platforms for the immobilization of molecular species. In the present research, factors affecting adsorption and desorption of propidium iodide (PI) dye, chosen as a charged molecule model, on ND and sp 2 carbon nanoparticles were studied, with a size ranging from 75 to 4,305 nm. It was found that adsorption of PI molecules, as characterized by ultraviolet–visible spectroscopy, on ND particles is strongly influenced by sorbent-sorbate electrostatic interactions. Different types of NDs with a negative zeta potential were found to adsorb positively charged PI molecules, while no PI adsorption was observed for NDs with a positive zeta potential. The type and density of surface groups of negatively charged NDs greatly influenced the degree and capacity of the PI adsorbed. Ozone-purified NDs had the highest capacity for PI adsorption, due to its greater density of oxygen containing groups, i.e., acid anhydrides and carboxyls, as assessed by TDMS and TOF–SIMS. Single wall nanohorns and carbon onion particles were found to adsorb PI regardless of their zeta potential; this is likely due to π bonding between the aromatic rings of PI and the graphitic surface of the materials and the internal cavity of the horns.






Similar content being viewed by others
Notes
MOPAC2009, Stewart, J.J.P. Stewart Computational Chemistry, Version 8.318 M web: http://OpenMOPAC.net.
References
Bakowicz K, Mitura S (2002) Biocompatibility of NCD. J Wide Bandgap Mater 9(4):261–272
Chao JI, Perevedentseva E, Chung PH, Liu KK, Cheng CY, Chang CC, Cheng CL (2007) Nanometer-sized diamond particle as a probe for biolabeling. Biophys J 93(6):2199–2208
Chen M, Pierstorff ED, Lam R, Li SY, Huang H, Osawa E, Ho D (2009) Nanodiamond-mediated delivery of water-insoluble therapeutics. ACS Nano 3(7):2016–2022
Chiganova GA (2000) Aggregation of particles in ultradispersed diamond hydrosols. Colloid J 62:238–243
Chow EK, Zhang X-Q, Chen M, Lam R, Robinson E, Huang H, Schaffer D, Osawa E, Goga A, Ho D (2011) Nanodiamond therapeutic delivery agents mediate enhanced chemoresistant tumor treatment. Sci Transl Med 3(73):1–11
Chung PH, Perevedentseva E, Tu JS, Chang CC, Cheng CL (2005) Spectroscopic study of bio-functionalized nanodiamonds. Diam Relat Mater 15(4–8):622–625
Cunningham G, Panich AM, Shames AI, Petrov I, Shenderova O (2008) Ozone-modified detonation nanodiamonds. Diam Relat Mater 17(4–5):650–654
Foglieni C, Meoni C, Davalli AM (2001) Fluorescent dyes for cell viability: an application on prefixed conditions. Histochem Cell Biol 115(3):223–229
Gibson N, Shenderova O, Puzyr A, Purtov K, Grichko V, Luo TJM, Fitzgerald Z, Bondar V, Brenner D (2007) Nanodiamonds for detoxification. In: NSTI nanotechnology conference and trade show–NSTI nanotech technical proceedings, vol 2, Nanotech, Santa Clara, pp. 713–716
Gibson N, Shenderova O, Luo TJM, Moseenkov S, Bondar V, Puzyr A, Purtov K, Fitzgerald Z, Brenner DW (2009) Colloidal stability of modified nanodiamond particles. Diam Relat Mater 18(4):262–620
Gibson NM, Luo TJM, Shenderova O, Choi YJ, Brenner DW (2010a) Modified nanodiamonds for adsorption of propidium iodide and aflatoxin. MRS Fall 2009 Proceedings, available online, Paper #1236-SS09-05
Gibson NM, Luo TJM, Shenderova O, Choi YJ, Fitzgerald Z, Brenner DW (2010b) Fluorescent dye adsorption on nanocarbon substrates through electrostatic. Interactions 19(2–3):234–237
Giles CH, Smith D, Huitson A (1974) A general treatment and classification of the solute adsorption isotherm. I. Theoretical. J Colloid Interf Sci 47(3):755–765
Gitig DM, Koff A (2001) Cdk pathway: cyclin-dependent kinases and cyclin-dependent kinase inhibitors. Mol Biotechnol 19(2):179–188
Grichko V, Grishko V, Shenderova O (2006) Nanodiamond bullets and their biological targets. NanoBioTechnol 2(1–2):1294–1551
Hens S, Cunningham G, Tyler T, Moseenkov S, Kuznetsov V, Shenderova O (2008) Nanodiamond bioconjugate probes and their collection by electrophoresis. Diam Relat Mater 17(11):1858–1866
Huang LCL, Chang HC (2004) Adsorption and immobilization of cytochrome c on nanodiamonds. Langmuir 20(14):5870–5884
Huang TS, Tzeng Y, Liu YK, Chen YC, Walker KR, Guntupalli R, Liu C (2004) Immobilization of antibodies and bacterial binding on nanodiamond and carbon nanotubes for biosensor applications. Diam Relat Mater 13(4–8):1098–1102
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14(1):33–38
Kossovsky N, Gelman A, Hnatyszyn HJ, Rajguru A, Garrell RL, Torbati S, Freitas SSF, Chow MG (1995) Surface-modified diamond nanoparticles as antigen delivery vehicles. Bioconjugugate Chem 6(5):507–511
Krueger A (2007) New carbon materials: biological applications of functionalized nanodiamond materials. Chem A Eur J 14(5):1382–1390
Liu KK, Cheng CL, Chang CC, Chao JI (2007) Biocompatible and detectable carboxylated nanodiamond on human cell. Nanotechnology 18(32):325102
Menozzi FD, Michel A, Pora H, Miller AOA (1990) Absorption method for rapid decontamination of solutions of ethidium bromide and propidium iodide. Chromatographia 29(3–4):167–169
Mohan N, Chen CS, Hsieh HH, Wu YC, Chang HC (2010) In vivo imaging and toxicity assessments of fluorescent nanodiamonds in Caenorhabditis elegans. Nano Lett 10(9):3692–3699
Petrov I, Shenderova O, Grishko V, Grichko V, Tyler T, Cunningham G, McGuire G (2007) Detonation nanodiamonds simultaneously purified and modified by gas treatment. Diam Relat Mater 16(12):2098–2103
Puzyr AP, Bondar VS, (2003) Method of production of nanodiamonds of explosive synthesis with an increased colloidal stability, St. Petersburg
Puzyr AP, Pozdniakova IO, Bondar VS (2004) Design of a luminescent biochip with nanodiamonds and bacterial luciferase. Phys Solid State 46(4):761–763
Puzyr AP, Baron AV, Purtov KV, Bortnikov EV, Skobelev NN, Mogilnaya OA, Bondar VS (2007a) Nanodiamonds with novel properties: a biological study. Diam Relat Mater 16(12):2124–2128
Puzyr AP, Purtov KV, Shenderova OA, Luo M, Brenner DW, Bondar VS (2007b) The adsorption of aflatoxin B1 by detonation-synthesis nanodiamonds. Doklady Biochem Biophys 417(1):299–301
Puzyr AP, Burov AE, Bondar VS, Trusov YN (2010) Neutralization of aflatoxin B1 by ozone treatment and adsorption by nanodiamonds. Nanotechnol Russ 5(1–2):137–141
Raina S, Kang WP, Davidson JL (2010) Nanodiamond macro- and microelectrode array bio-sensor. IEEE Sens Conf 2009:1780–1783
Schmid I, Krall WJ, Uittenbogaart CH, Braun J, Giorgi V (1992) Dead cell discrimination with 7-amino-actinomycin D in combination with dual color immunofluorescence in single laser flow cytometry. Cytom Part A 13(2):204–208
Schrand AM, Huang HJ, Carlson C, Schlager J, Osawa E, Hussain S, Dai L (2007a) Are diamond nanoparticles cytotoxic? J Phys Chem B 111(1):2–7
Schrand AM, Dai L, Schlager JJ, Hussain SM, Osawa E (2007b) Differential biocompatibility of carbon nanotubes and nanodiamonds. Diam Relat Mater 16(2):2118–2123
Schrand AM, Johnson J, Dai L, Hussain SM, Schlager JJ, Zhu L, Hong Y, Osawa E (2008) Cytotoxicity and genotoxicity of carbon nanomaterials. In: Webster T (ed) Safety of nanoparticles: from manufacturing to clinical applications. Springer, Providence
Schrand AM, Hens SAC, Shenderova OA (2009) Nanodiamond particles: properties and perspectives for bioapplications. Crit Rev Solid State Mater Sci 34:18–74
Shenderova OA, Hens SAC (2010) Detonation nanodiamond particles processing, modification and bioapplications, In Ho D (ed) Nanodiamonds: applications in biology and nanoscale medicine, Springer, New York, pp 79–116
Turner S, Lebedev OI, Shenderova O, Vlasov II, Verbeeck J, Van Tendeloo G (2009) Determination of size, morphology, and nitrogen impurity location in treated detonation nanodiamond by transmission electron microscopy. Adv Funct Mater 19(13):2116–2124
Ushizawa K, Sato Y, Mitsumory T, Machinami T, Ueda T, Ando T (2002) Covalent immobilization of DNA on diamond and its verification by diffuse reflectance infrared spectroscopy. Chem Phys Lett 351(1–2):105–108
Vaijayanthimala V, Tzeng YK, Chang HC, Li CL (2009) The biocompatibility of fluorescent nanodiamonds and their mechanism of cellular uptake. Nanotechnology 20(42):425103
Waring MJ (1965) Complex formation between ethidium bromide and nucleic acids. J Mol Biol 13(1):269–282
Xing Y, Dai L (2009) Nanodiamonds for nanomedicine. Nanomedicine 4(2):207–218
Yang W, Auciello O, Butler JE, Cai W, Carlisle JA, Gerbi JE, Gruen DM, Knickerbocker T, Lasserter TL, Russell JN, Smith LM, Hamers RJ (2002) DNA-modified nanocrystalline diamond thin-films as stable, biologically active substrates. Nat Mater 1:253–257
Yeap WS, Tan YY, Loh KP (2008) Using Detonation nanodiamond for the specific capture of glycoproteins. Anal Chem 80(12):4659–4665
Yu SJ, Kang MW, Chang HC, Chen KM, Yu YC (2005) Bright fluorescent nanodiamonds: no photobleaching and low cytotoxicity. J Am Chem Soc 127(50):17604–17605
Yuan Y, Chen Y, Liu JH, Wang H, Liu Y (2009) Biodistribution and fate of nanodiamonds in vivo. Diam Relat Mater 18(1):95–100
Zhu Y, Li W, Li Q, Li Y, Li Y, Zhang X, Huang Q (2009) Effects of serum proteins on intracellular uptake and cytotoxicity of carbon nanoparticles. Carbon 47(5):1351–1358
Acknowledgments
This research is supported by the Materials World Network program of the National Science Foundation under Grant No DMR-0602906. O.S. acknowledges the partial support through Air Force Office of Scientific Research under grant N66001-04-1-8933. In addition, we thank V. Kuznetsov, of the Boreskov Institute of Catalysis, Novosibirsk for providing onion-like carbon samples, V. Vorobyev for providing NDW− samples, Yury Gogotsi, Department of Materials Science and Engineering and Nanomaterials Group at Drexel University, for the BET analysis, Elaine Chuanzhen Zhou at the Analytical Instrumentation Facility for TOF–SIMS experiments and Zachary Fitzgerald, Department of Materials Science and Engineering at North Carolina State University for his modeling of the PI molecule. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gibson, N.M., Luo, TJ.M., Shenderova, O. et al. Electrostatically mediated adsorption by nanodiamond and nanocarbon particles. J Nanopart Res 14, 700 (2012). https://doi.org/10.1007/s11051-011-0700-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-011-0700-9