Abstract
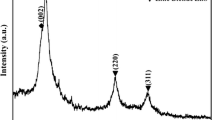
ZnO nanostructures including nanorods, dense, and partially hollow spheres were synthesized via a solution synthesis method with temperature ranging from 65 to 95 °C. Scanning electron microscopy (SEM) revealed that the diameter of the spheres is in the range of 200–500 nm. Transmission electron microscopy (TEM) showed that some of the spheres are hollow or partially hollow. Powder X-ray Diffraction (XRD) and TEM-Selected area electron diffraction (SAED) analysis showed that the spheres consist of polycrystalline nanoparticles. It was found for the first time that the agitation during the synthesis plays a critical role on morphology of the ZnO nanostructures formed in solution. The oriented attachment of nanocrystals without agitation during the synthesis could guide the nanocrystals to form an ordered nanorod structure. However, the disordered aggregation of the nanocrystals under shear force resulted in a spherical morphology. It was also found that the composition of spheres is different from that of nanorods: the spheres consist of both ZnO and Zn(OH)2, but nanorods consist of single-crystal ZnO only. Zn(OH)2 presented in the spheres could decompose to ZnO by calcination, resulting in the formation of hollow spheres.








Similar content being viewed by others
References
Beek WJE, Wienk MM, Janssen RAJ (2004) Efficient hybrid solar cells from zinc oxide nanoparticles and a conjugated polymer. Adv Mater 16(12):1009–1013
Cao BQ, Matsumoto T, Matsumoto M, Higashihata M, Nakamura D, Okada T (2009) ZnO nanowalls grown with high-pressure PLD and their applications as field emitters and UV detectors. J Phys Chem C 113(25):10975–10980
Caruso F, Caruso RA, Mohwald H (1998) Nanoengineering of inorganic and hybrid hollow spheres by colloidal templating. Science 282(5391):1111–1114
Chen H, Kou X, Yang Z, Ni W, Wang J (2008a) Shape- and size-dependent refractive index sensitivity of gold nanoparticles. Langmuir 24(10):5233–5237
Chen HM, Liu RS, Lo MY, Chang SC, Tsai LD, Peng YM, Lee JF (2008b) Hollow platinum spheres with nano-channels: synthesis and enhanced catalysis for oxygen reduction. J Phys Chem C 112(20):7522–7526
Davis ME (2002) Ordered porous materials for emerging applications. Nature 417(6891):813–821
Deng ZW, Chen M, Gu GX, Wu LM (2008) A facile method to fabricate ZnO hollow spheres and their photocatalytic property. J Phys Chem B 112(1):16–22
Greyson EC, Babayan Y, Odom TW (2004) Directed growth of ordered arrays of small-diameter ZnO nanowires. Adv Mater 16(15):1348–1352
Huang SS, Fan Y, Cheng ZY, Kong DY, Yang PP, Quan ZW, Zhang CM, Lin J (2009) Magnetic mesoporous silica spheres for drug targeting and controlled release. J Phys Chem C 113(5):1775–1784
Jiang P, Bertone JF, Colvin VL (2001) A lost-wax approach to monodisperse colloids and their crystals. Science 291(5503):453–457
Kamata K, Lu Y, Xia YN (2003) Synthesis and characterization of monodispersed core-shell spherical colloids with movable cores. J Am Chem Soc 125(9):2384–2385
Kidambi S, Lee I, Chan C (2004) Controlling primary hepatocyte adhesion and spreading on protein-free polyelectrolyte multilayer films. J Am Chem Soc 126(50):16286–16287
Kim SW, Kim M, Lee WY, Hyeon T (2002) Fabrication of hollow palladium spheres and their successful application to the recyclable heterogeneous catalyst for Suzuki coupling reactions. J Am Chem Soc 124(26):7642–7643
Kong XY, Ding Y, Yang R, Wang ZL (2004) Single-crystal nanorings formed by epitaxial self-coiling of polar nanobelts. Science 303(5662):1348–1351
Li Z, Yang RS, Yu M, Bai F, Li C, Wang ZL (2008) Cellular level biocompatibility and biosafety of ZnO nanowires. J Phys Chem C 112(51):20114–20117
Li QY, Wang EB, Li SH, Wang CL, Tian CG, Sun GY, Gu JM, Xu R (2009a) Template-free polyoxometalate-assisted synthesis for ZnO hollow spheres. J Solid State Chem 182(5):1149–1155
Li XF, Lv KL, Deng KJ, Tang JF, Su R, Sun J, Chen LQ (2009b) Synthesis and characterization of ZnO and TiO2 hollow spheres with enhanced photoreactivity. Mater Sci Eng B 158(1–3):40–47
Lucas M, Mai W, Yang R, Wang ZL, Riedo E (2007) Aspect ratio dependence of the elastic properties of ZnO nanobelts. Nano Lett 7(5):1314–1317
Meng X, Lin B, Gu B, Zhu J, Fu Z (2005) A simple growth route towards ZnO thin films and nanorods. Solid State Commun 135(7):411–415
Nakashima T, Kimizuka N (2003) Interfacial synthesis of hollow TiO2 microspheres in ionic liquids. J Am Chem Soc 125:6368–6387
Navale SC, Gosavi SW, Mulla IS (2008) Controlled synthesis of ZnO from nanospheres to micro-rods and its gas sensing studies. Talanta 75(5):1315–1319
Pan ZW, Dai ZR, Wang ZL (2001) Nanobelts of semiconducting oxides. Science 291(5510):1947–1949
Penn RL, Banfield JF (1998) Imperfect oriented attachment: dislocation generation in defect-free nanocrystals. Science 281(5379):969–971
Qin Y, Wang XD, Wang ZL (2008) Microfibre-nanowire hybrid structure for energy scavenging. Nature 451(7180):809–813
Sun Y, Zhuang L, Lu J, Hong X, Liu P (2007) Collapse in crystalline structure and decline in catalytic activity of Pt nanoparticles on reducing particle size to 1 nm. J Am Chem Soc 129(50):15465–15467
Vayssieres L (2003) Growth of arrayed nanorods and nanowires of ZnO from aqueous solutions. Adv Mater 15(5):464–466
Wang ZL, Song J (2006) Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science 312(5771):243–246
Wang W, Zeng B, Yang J, Poudel B, Huang J, Naughton MJ, Ren Z (2006) Aligned ultralong ZnO nanobelts and their enhanced field emission. Adv Mater 18(24):3275–3278
Wang XD, Song JH, Liu J, Wang ZL (2007) Direct-current nanogenerator driven by ultrasonic waves. Science 316(5821):102–105
Weintraub B, Deng YL, Wang ZL (2007) Position-controlled seedless growth of ZnO nanorod arrays on a polymer substrate via wet chemical synthesis. J Phys Chem C 111(28):10162–10165
Weintraub B, Chang S, Singamaneni S, Han W, Choi YJ, Bae J, Kirkham M, Tsukruk V, Deng Y (2008) Density-controlled, solution-based growth of ZnO nanorod arrays via layer-by-layer polymer thin films for enhanced field emission. Nanotechnology 19:435302–435308
Wijnhoven J, Vos WL (1998) Preparation of photonic crystals made of air spheres in titania. Science 281(5378):802–804
Xu S, Lao C, Weintraub B, Wang ZL (2008a) Density-controlled growth of aligned ZnO nanowire arrays by seedless chemical approach on smooth surfaces. J Mater Res 23(8):2072–2077
Xu S, Wei Y, Kirkham M, Liu J, Mai W, Davidovic D, Snyder RL, Wang ZL (2008b) Patterned growth of vertically aligned ZnO nanowire arrays on inorganic substrates at low temperature without catalyst. J Am Chem Soc 130(45):14958–14959
Yang HG, Zeng HC (2004) Preparation of hollow anatase TiO2 nanospheres via Ostwald ripening. J Phys Chem B 108(11):3492–3495
Zhang J, Wang SR, Wang Y, Xu MJ, Xia HJ, Zhang SM, Huang WP, Guo XZ, Wu SH (2009) ZnO hollow spheres: preparation, characterization, and gas sensing properties. Sens Actuat B 139(2):411–417
Zhong ZY, Yin YD, Gates B, Xia YN (2000) Preparation of mesoscale hollow spheres of TiO2 and SnO2 by templating against crystalline arrays of polystyrene beads. Adv Mater 12(3):206–209
Zhu YF, Shi JL, Shen WH, Dong XP, Feng JW, Ruan ML, Li YS (2005) Stimuli-responsive controlled drug release from a hollow mesoporous silica sphere/polyelectrolyte multilayer core-shell structure. Angew Chem Int Ed 44(32):5083–5087
Acknowledgments
We would like to thank IPST at Georgia Tech for the financial support to the project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, Z., Ding, Y., Zu, X. et al. ZnO spheres and nanorods formation: their dependence on agitation in solution synthesis. J Nanopart Res 13, 1689–1696 (2011). https://doi.org/10.1007/s11051-010-9922-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-010-9922-5