Abstract
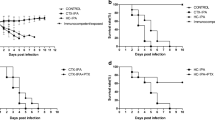
Paracoccidioidomycosis (PCM) is a granulomatous disease caused by a dimorphic fungus, Paracoccidioides brasiliensis (Pb). To determine the influence of nitric oxide (NO) on this disease, we tested cis-[Ru(bpy)2(NO)SO3](PF6), ruthenium nitrosyl, which releases NO when activated by biological reducing agents, in BALB/c mice infected intravenously with Pb 18 isolate. In a previous study by our group, the fungicidal activity of ruthenium nitrosyl was evaluated in a mouse model of acute PCM, by measuring the immune cellular response (DTH), histopathological characteristics of the granulomatous lesions (and numbers), cytokines, and NO production. We found that cis-[Ru(bpy)2(NO)SO3](PF6)-treated mice were more resistant to infection, since they exhibited higher survival when compared with the control group. Furthermore, we observed a decreased influx of inflammatory cells in the lung and liver tissue of treated mice, possibly because of a minor reduction in fungal cell numbers. Moreover, an increased production of IL-10 and a decrease in TNF-α levels were detected in lung tissues of infected mice treated with cis-[Ru(bpy)2(NO)SO3](PF6). Immunohistochemistry showed that there was no difference in the number of VEGF- expressing cells. The animals treated with cis-[Ru(bpy)2(NO)SO3](PF6) showed high NO levels at 40 days after infection. These results show that NO is effectively involved in the mechanism that regulates the immune response in lung of Pb-infected mice. These data suggest that NO is a resistance factor during paracoccidioidomycosis by controlling fungal proliferation, influencing cytokine production, and consequently moderating the development of a strong inflammatory response.








Similar content being viewed by others
References
Restrepo A, Tobo′n AM. Paracoccidioides brasilensis. In: Mandell GL, Bennet JE, Dollin R, editors. Principles and practice of infectious diseases. Philadelphia: Elsevier; 2005. p. 3062–8.
Borges-Walmsley MI, Chen D, Shu X, Walmsley AR. The pathobiology of Paracoccidioides brasiliensis. Trends Microbiol. 2002;10:80–7.
Mamoni RL, Blotta MHSL. Kinetics of cytokines and chemokines gene expression distinguishes Paracoccidioides brasiliensis infection from disease. Cytokine. 2005;32:20–9.
Pulendran B. Modulating Th1/Th2 responses with microbes, dendritic cells, and pathogen recognition receptors. Immunol Res. 2004;29:187–96.
Corthay A. A three-cell model for activation of naïve T helper cells. Scand J Immunol. 2006;64:93–6.
Gonzalez A, De Gregori W, Velez D, Restrepo A, Cano LE. Nitric oxide participation in the fungicidal mechanism of interferon-activated murine macrophages against Paracoccidioides brasiliensis conidia. Infect Immun. 2000;68:2546–52.
MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, Stevens K, Xie Q, Sokol K, Hutchinson N, Chen H, Mudgett JS. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641.
Liew FY, Millot S, Parkinson C, Palmer RMJ, Moncada S. Macrophage killing of Leishmania parasitic in vivo is mediated by nitric oxide from l-arginine. J Immunol. 1990;144:4794–7.
James SL, Glaven J. Macrophage citotoxicity against schistosomula of Schistosoma mansoni involved arginine-dependent production of reactive nitrogen intermediates. J Immunol. 1989;143:4208–12.
Lane TE, Otero GC, Wa-Hsieh B, Howard D. Expression of inducible nitric oxide synthase by stimulated macrophages correlates with their antihistoplasma activity. Infect Immun. 1994;62:1940–5.
Bocca AL, Hayashi EE, Pinheiro AG, Furlanetto AB, Campanelli AP, Cunha FQ, Figueiredo F. Treatment of Paracoccidioides brasiliensis-infected mice with a nitric oxide inhibitor prevents the failure of cell-mediated immune response. J Immunol. 1998;161:3056.
Nascimento FRF, Calich VLG, Rodríguez D, Russo M. Dual role for nitric oxide in paracoccidioidomycosis: essential for resistance, but overproduction associated with susceptibility. J Immunol. 2002;168:4593–600.
Bogdan C. Nitric oxide and the immune response. Nat Immun. 2001;10:907–16.
Zanichelli PG, Sernaglia RL, Franco DW. Immobilization of the [RuII(edta)NO+] íon on surface of functionalized sílica gel. Langmuir. 2006;22:203–8.
Sánchez-Delgado RA, Navarro M, Lazardi K, Atencio R, Capparelli M, Vargas F, Urbina JA, Bouillez A, Noels AF, Mais D. Toward a novel metal-based chemotherapy against tropical diseases. Part 4. Synthesis and characterization of new metal–clotrimazole complexes and evaluation of their activity against trypanosoma cruzi. Inorg Chim Acta. 1998;39:528–540.
Navarro MT, Lahmann EJ, Cisneros-Fajardo A, Fuentes RA, Sánchez-Delgado P, Silva JA. Toward a novel metal-based chemotherapy against tropical diseases. Part 5. Synthesis and characterization of new Ru(II) and Ru(III) clotrimazole and ketoconazole complexes and evaluation of their activity against trypanosoma cruzi polyhedron. 2000;19:2319–2325.
Sanchez-Delgado RA, Anzellotti A. Metal complexes as chemotherapeutic agents against tropical diseases: trypanosomiasis, malaria and leishmaniasis mini. Rev Med Chem. 2004;4:23–30.
Tfouni E, Krieger M, McGarvey B, Franco DW. Structure, chemical and photochemical reactivity and biological activity of some ruthenium nitrosyl complexes. Coord Chem Rev. 2003;236:57–69.
Silva JJN, Osakabe AL, Pavanelli WR, Silva JS, Franco DW. In vitro and in vivo antiproliferative and trypanocidal activities of ruthenium NO donors Br. J Pharmacol. 2007;152:112–21.
Calich VLG, Purchio A, Paulo CR. A new fluorescent viability test for fungi cells. Mycopathologia. 1979;66:175–7.
Souto JT, Figueiredo F, Furlanetto A, Pfeffer K, Rossi MA, Silva JS. IFN-γ and TNF-α determine resistance to Paracoccidioides brasiliensis infection in mice. Am J Pathol. 2000;156:811–1820.
Perrin DD, Armarego WLF, Perrin DR. Purification of laboratory chemicals. Elmsford, USA: Pergamon Press; 1980.
Shriver DF. The manipulation of air-sensitive compound. McGraw-Hill: New York; 1969.
Borges SSS, Davanzo CU, Castellano EE, Z-Zchpector J, Silva SC, Franco DW. Ruthenium nitrosyl complexes with N-heterocyclic ligands. Inorg Chem. 1998;37:2670–7.
Cerecetto H, González M. Chemotherapy of Chagas’ disease: status and new development. Curr Top Med Chem. 2002;2:1187–213.
Clarke MJ. Electrochemistry, synthesis, and spectra of pentaammineruthenium(III) complexes of cytidine, adenosine, and related ligands. J Am Chem Soc. 1978;100:5068–75.
Panis C, Mazzuco TL, Costa CZ, Victorino VJ, Tatakihara VL, Yamauchi LM, Yamada-Ogatta SF, Cecchini R, Rizzo LV, Pinge-Filho P. Trypanosoma cruzi: effect of the absence of 5-lipoxygenase (5-LO)-derived leukotrienes on levels of cytokines, nitric oxide and iNOS expression in cardiac tissue in the acute phase of infection in mice. Exp Parasitol. 2010. (In press).
Mariano FS, Gutierrez FR, Pavanelli WR, Milanezi CM, Cavassani KA, Moreira AP, Ferreira BR, Cunha FQ, Cardoso CR, Silva JS. The involvement of CD4+CD25+ T cells in the acute phase of Trypanosoma cruzi infection. Microbes Infect. 2008;10:825–33.
Furuta T, Kimura M, Watanabe N. Elevated levels of vascular endothelial growth factor (VEGF) and soluble vascular endothelial growth factor receptor (VEGFR)-2 in human malaria. Am J Trop Med Hyg. 2010;82:136–9.
Vespa GNR, Cunha FQ, Silva JS. Nitric oxide is involved in control of Trypanosoma cruzi-induced parasitemia and directly kills the parasite in vitro. Infect Immun. 1994;62:5177.
Wei X, Charles IG, Smith A, Ure J, Feng GJ, Huang FP, Xu D, Muller W, Moncada S, Liew FY. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408.
Candolfi E, Hunter CA, Remington JS. Mitogen- and antigen-specific proliferation of T-cells in murine toxoplasmosis is inhibited by reactive nitrogen intermediates. Infect Immun. 1994;62:1995.
Gregory SH, Wing EJ, Hoffman RA, Simmons RL. Reactive nitrogen intermediates suppress the primary immunologic response to Listeria. J Immunol. 1993;150:2901.
Rockett KA, Auburu MM, Rockett EJ, Coride WB, Clark I. Possible role of nitric oxide in malarial immunosuppression. Parasite Immunol. 1994;16:243.
Teixeira HC, Calich VLG, Singer-Vermes LM, D’Império Lima MR, Russo M. Experimental paracoccidioidomycosis: early immunosuppression occurs in susceptible mice after infection with pathogenic fungi. Braz J Med Biol Res. 1987;20:587.
Tonnetti L, Spaccapelo R, Cenci E, Mencacci A, Puccetti P, Coffman RL, Bistoni F, Romani L. Interleukin-4 and 10 exacerbate candidiasis in mice. Eur J Immunol. 1995;25:1559–65.
Del Sero G, Mencacci A, Cenci E, d’Ostiani CF, Montagnoli C, Bacci A, Mosci P, Kopf M, Romani L. Antifungal type 1 response are upregulated in IL-10-deficient mice. Microbes Infect. 1999;1:1169–80.
Vazquez-Torres J, Jones-Carson RD, Wagner T, Balish E. Early resistance of interleukin-10 knockout mice to acute systemic candidiasis. Infect Immun. 1999;67:670–4.
Deepe GS, Gibbons RS. Protective and memory immunity to Histoplasma capsulatum in the absence of IL-10. J Immunol. 2003;171:5353–62.
Fierer J, Walls L, Eckmann L, Yamamoto T, Kirkland TN. Importance of interleukin-10 in genetic susceptibility of mice to Coccidioides immitis. J Immunol. 1998;66:4397–402.
Ohba T, Haro H, Ando T, Wako M, Suenaga F, Aso Y, Koyama K, Hamada Y, Nako A. TNF-alpha-induced NF-kappaB signaling reverses age-related declines in VEGF induction and angiogenic activity in intervertebral disc tissues. J Orthop Res. 2009;27(2):229–35.
Kim JE, Son JE, Jung SK, Kang NJ, Lee CY, Lee KW, Lee HJ. Cocoa polyphenols suppress TNF-alpha-induced vascular endothelial growth factor expression by inhibiting phosphoinositide 3-kinase (PI3 K) and mitogen-activated protein kinase-1 (MEK1) activities in mouse epidermal cells. Br J Nutr. 2010;16:1–8.
Zhen G, Xue Z, Zhao J, Gu N, Tang Z, Xu Y, Zhang Z. Mesenchymal stem cell transplantation increases expression of vascular endothelial growth factor in papain-induced emphysematous lungs and inhibits apoptosis of lung cells. Cytotherapy. 2010;29.
Abbas AK, Lichtman AH. Citocinas, Imunologia celular e molecular. 5th ed. Brazil: Elsevier; 2005. p. 260–2. pp. 270, 276–278.
Guedes PM, Oliveira FS, Gutierrez FR, da Silva GK, Rodrigues GJ, Bendhack LM, Franco DW, Do Valle Matta MA, Zamboni DS, da Silva RS, Silva JS. Nitric oxide donor trans-[RuCl([15]aneN)NO] as a possible therapeutic approach for Chagas’ disease. Br J Pharmacol. 2010;160(2):270–82.
Hesse M, Modolell M, La Flamme AC, Schito M, Fuentes JM, Cheever AW, Pearce EJ, Wynn TA. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by pattern of l-argininie metabolism. J Immunol. 2001;167:6533–44.
Lousada S, Flórido M, Appelberg R. Regulation of granuloma fibrosis by nitric oxide during Mycobacterium avium experimental infection. Int J Exp Pathol. 2006;87:307–15.
Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516.
Brummer E. Interaction of Paracoccidioides brasiliensis with host defense cells. In: Franco M, Silva Lacaz C, Restrepo Moreno A, del Negro, editors. Paracoccidioidomycosis. Boca Raton: CRC Press; 1994. p. 213–223.
Fricker SP. Ruthenium, nitric oxide and disease. Platinum Metal Rev. 1995;59:150–9.
Acknowledgments
The authors would like to acknowledge the financial support from Fundação Araucária/SETI-PR, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Special Program for Research and Training in Tropical Diseases (TDR/WHO). Dr. A. Leyva provided English editing of the manuscript.
Conflict of Interest
The authors have no financial conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is based on part of the post-doctoral work of WR Pavanelli.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pavanelli, W.R., da Silva, J.J.N., Panis, C. et al. Experimental Chemotherapy in Paracoccidioidomycosis Using Ruthenium NO Donor. Mycopathologia 172, 95–107 (2011). https://doi.org/10.1007/s11046-011-9416-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-011-9416-8