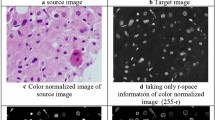
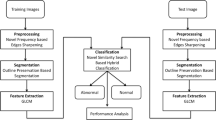
Abstract
The differentiation of a cluster of nuclei and multi-nucleation is a critical issue in automated diagnosis systems. Due to the similarities between said clusters and malignant nuclei, misclassification of these regions can affect the automated systems’ final decision. In this paper, a method for differentiating clusters from multi nucleated cells in histopathological images is proposed. Hepatocellular Carcinoma(HCC) and Dysplasia are characterized by cellular and nuclear enlargement, nuclear pleomorphism and multinucleation, which possess prominent threat Data was obtained from Global Hospital and Research Center from patients diagnosed with Hepatocellular Carcinoma and Dysplasia. This paper introduces a hybrid diagnosis method that uses texture, layout and context features of nuclei and cytoplastic cells in order to enhance the poor diagnosis of liver tumors in Infra Red (IR) images. We propose a Area based Adaptive Expectation Maximization(EM) that grows the clusters, which avoids the need for initial cluster selection in order to obtain texton maps of nuclei and cytoplasm. A linear regression model of nuclei and cytoplastic changes were built by incorporating the aforementioned features efficiently. The proposed method provides better classification and segmentation accuracy of nuclei and extra nuclear content in HCC and dysplasia, compared to the state-of-the-art methods like convolutional networks and classical methods like Adaptive K means and EM method in constant time. In conclusion, this system detects the malignant cells and the highly eligible precancerous cells which is cost effective and reproducible.










Similar content being viewed by others
References
Anthony PP, Vogel CL, Barker LE (1973) Liver cell dysplasia: a premalignant condition. J clin Path 26(3):217–223
Arakawa M, Kage M, Sugihara S et al (1986) Emergence of malignant lesions within an adenomatous hyperplastic nodule in a cirrhotic liver. Observations in five cases. Gastroenterology 91(1):198–208
Bani MR, Lux MP, Heusinger K et al (2009) Factors correlating with reexcision after breast-conserving therapy. Eur J Surg Oncol 35(1):32–37. doi:10.1016/j.ejso.2008.04.008
Basu M (2002) Gaussian-based edge-detection methods-a survey. IEEE Transactions on Systems, Man and Cybernetics-Part C: Applications And Reviews 32(3):252–260
Bhagwati CP, Sinha GR (2010) An adaptive K-means clustering algorithm for breast image segmentation. Int J of Computer Applications 10(4):35–38
Bhatia SK (2004) Adaptive K-means clustering. FLAIRS Conference p:695–699
Bruix J, Branco FS, Ayuso C (2006) Hepatocellular Carcinoma. In: Schiff ER, Sorrel MF, Maddrey WC (eds) Schiff’s diseases of liver, 10th edn. hippincott Williams and Wilkins, Philadelphia
Casciaro S, Franchini R, Massoptier L et al (2012) Fully automatic segmentations of liver and hepatic tumors from 3-D computed tomography abdominal images: comparative evaluation of two automatic methods. IEEE Sensors J 12(3):464–473
Chen Z, Butke R, Miller B et al (2013) Infrared metrics for fixation-free liver tumor detection. J Phys Chem B 117(41). doi:10.1021/jp4073087
Duygulu P, Barnard K, de Freitas JFG et al (2002) Object recognition as machine translation: learning a lexicon for a fixed image vocabulary. Proc European Conf on Computer Vision Springer-Verlag Berlin Heidelberg 2353:97–112
Fernandez DC, Bhargava R, Hewitt SM et al (2005) Infrared spectroscopic imaging for histopathologic recognition. Nat Biotech 23:469–474. doi:10.1038/nbt1080
Freund Y, Schapire RE (1999) A short introduction to boosting. J Japanese Society for Artificial Intelligence 14(5):771–780
Friedman J, Hastie T, Tibshirani R (2000) Additive logistic regression: a statistical view of boosting. Ann Statistics 28(2):337–407
Hytiroglou P (2004) Morphological changes of early human hepatocarcinogenesis. Semin Liver Dis 24(1):65–75
Hytiroglou P, Park YN, Krinsky G, Theise ND (2007) Hepatic precancerous lesions and small hepatocellular carcinoma. Gastroenterol Clin N Am 36(4):867–887
International Consensus Group for Hepatocellular Neoplasia (2009) Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology 49(2):658–664
Konishi S, Yuille AL (2000) Statistical cues for domain specific image segmentation with performance analysis. Proc. IEEE Conf. Computer vision and. Pattern Recogn 1:125–132
Kuang Z, Schnieders D, Zhou H et al (2012) Learning image-specific parameters for interactive segmentation. IEEE Conference on Computer Vision and Pattern Recognition:590–597
Lafferty J, McCallum A, Pereira F (2001) Conditional random fields: probabilistic models for segmenting and labeling sequence data. Proc Int Conf on Machine Learning:282–289
Jonathan Long, Evan Shelhamer and Trevor Darrell (2015) Fully Convolutional Networks for Semantic Segmentation. Proc. IEEE CVPR15
Jitendra Malik, Hariharan B, Pablo A, et al. (2015) Hypercolumns for Object Segmentation and Fine-grained Localization. Proc. IEEE CVPR15
Marchio A, Terris B, Meddeb M et al (2001) Chromosomal abnormalities in liver cell dysplasia detected by comparative genome hybridisation. Mol Pathol 54(4):270–274
Marengo A, Jouness RIK, Bugianesi E (2015) Progression and natural history of nonalcoholic fatty liver disease in adults. Clin Liver Dis doi. doi:10.1016/j.cld.2015.10.010
Moon N, Bullitt E, Leemput KV et al (2002) Model-based brain and tumor segmentation. IEEE 1:528–531
Nakanuma Y, Hirata K (1993) Unusual hepatocellular lesions in primary biliary cirrhosis resembling but unrelated to hepatocellular carcinoma. Virchows Arch A Path Anat 422(1):17–23
Plentz RR, Park YN, Lechel A et al (2007) Telomere shortening and p21 checkpoint inactivation characterize multistep hepatocarcinogenesis in humans. Hepatology 45(4):968–976
Porikli F (2005) Integral histogram: a fast way to extract histograms in Cartesian spaces. IEEE computer society conference on computer vision and. Pattern Recogn 1:829–836
Roberts LR, Gores GJ (2009) Hepatocellular Carcinoma. In: Yamada T (ed) Textbook of gastroenterology, vol 2, 5th edn. Blackwell, UK, Oxford, pp. 2386–2411
Rohit S, Gaikwad MS (2013) Segmentation of brain tumour and its area calculation in brain MR images using K-mean clustering and fuzzy C-mean algorithm. Int J of computer science and engineering. Technology 4(5):524
Russel BC, Torralba A, Murphy KP et al (2008) LabelMe: a database and webbased tool for image annotation. Int J of Computer Vision 77(1–3):157–173
Sakamoto M, Hirohashi S (1998) Natural history and prognosis of adenomatous hyperplasia to hepatocellular carcinoma: multi-institutional analysis of 53 nodules followed up for more than 6 months and 141 patients with single early hepatocellular carcinoma treated by surgical resection or percutaneous ethanol injection. Jpn J Clin Oncol 28(10):604–608
Schiller DE, Le LW, Cho BCJ et al (2008) Factors associated with negative margins of lumpectomy specimen: potential use in selecting patients for intraoperative radiotherapy. Ann Surg Oncol 15(3):833–842. doi:10.1245/s10434-007-9711-2
Shotton J, Winn J, Rother C, et al. (2006) TextonBoost: Joint Appearance, Shape and Context Modeling for Multi-Class Object Recognition and Segmentation. Proc. European Conf. on Computer Vision Springer-Verlag Berlin Heidelberg 3951: 1–15.
Takayama T, Makuuchi M, Hirohashi S et al (1990) Malignant transformation of adenomatous hyperplasia to hepatocellular carcinoma. Lancet 336(8724):1150–1153
Terminology of nodular hepatocellular lesions (1995) International working party. Hepatology 22(3):983–993
Tsai A, Zhang J, Willsky AS (2001) Expectation-maximization algorithms for image processing using multiscale models and mean-field theory, with applications to laser radar profiling and segmentation. Opt Eng 40(7):1287–1301
Tu Z, Chen X, Yuille AL et al (2005) Image parsing: unifying segmentation, detection, and recognition. Int J of Computer Vision 63(2):113–140
Acknowledgements
We would like to thank and extend a deep sense of gratitude to Dr. Mohamed Rela, MS FRCS, Consultant HPB Surgeon and Dr. Balajee, MD, HOD of Laboratory Medicine & Senior Consultant of Global Hospital, Chennai for providing the medical image data and interpretation for the analysis. They have helped us a lot in getting a better insight and assessing the number of liver metastases in histopathology images to a greater extent.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No. The study was approved by Institutional Ethics Committee-Global Hospital and Health City (IEC-GHHC).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Ethics Committee-Global Hospital and Health City(IEC-GHHC).
Rights and permissions
About this article
Cite this article
Kalinathan, L., Kathavarayan, R., Nagendram, D. et al. Segmentation of hepatocellular carcinoma and dysplastic liver tumors in histopathology images using area based adaptive expectation maximization. Multimed Tools Appl 77, 1761–1782 (2018). https://doi.org/10.1007/s11042-016-4260-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11042-016-4260-y