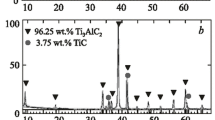
Treatment of initial powders of titanium, carbon and aluminum in a high-energy mill and pressure sintering are used for synthesizing dense and single-phase Ti2AlC and Ti3AlC2 ceramics. The effect of the content of aluminum and of the sintering temperature on the synthesis process is studied. During treatment in the high-energy mill the initial Ti, Al and C react and yield TiC and TiAl x . In the course of subsequent pressure sintering and depending on the content of aluminum, the milled powders interact and form TiC/Ti2AlC, Ti3AlC2,Ti2AlC, and Ti2AlC/TiAl.



Similar content being viewed by others
References
J. F. Zhu, J. Q. Gao, J. F. Yang, et al., J. Mater. Sci. Eng. A, 490, 62 – 65 (2008).
Z. J. Lin, M. J. Zhuo, Y. C. Zhou, et al., Acta Mater., 54, 1009 – 1015 (2006).
J. Y. Wang, Y. C. Zhou, Z. J. Lin, et al., Appl. Phys. Lett., 86 (2005).
Y. Khoptiar and I. Gotman, Mater. Lett., 57, 72 – 76 (2002).
W. B. Zhou, B. C. Mei, J. Q. Zhu, and X. L. Hong, Mater. Lett., 59, 131 – 134 (2005).
P. Wang, B. C. Mei, X. L. Hong, and W. B. Zhou, Trans. Nonferrous Met. Soc. China, 17, 1001 – 1004 (2007).
M. W. Barsoum, M. Ali, and T. El-raghy, Metall. Mater. Trans. A, 3, 1857 – 1865 (2000).
Y. C. Zhou and X. H. Wang, Mater. Res. Innov., 5, 87–93 (2001).
M. Pietzka and J. C. Schuster, J. Phase Equilib., 15, 392 – 400 (1994).
N. V. Tzenov and M. W. Barsoum, J. Am. Ceram. Soc., 83, 825 – 832 (2000).
X. H. Wang and Y. C. Zhou, J. Mater. Chem., 12, 455 – 460 (2002).
J. Q. Zhu, B. C. Mei, X. W. Xu, and J. Liu, Mater. Lett., 58, 588 – 592 (2004).
X. H. Wang and Y. C. Zhou, Acta Mater., 50, 3141 – 3149 (2002).
C. Q. Peng, C. A. Wang, and Y. Huang, Key Eng. Mater., 280 – 283, 1369 – 1372 (2005).
C. Yang, S. Z. Jin, B. Y. Liang, et al., J. Mater. Process. Technol., 209, 871 – 875 (2009).
X. H. Wang and Y. C. Zhou, Z. Metallkd., 93, 66 – 71 (2002).
M. W. Barsoum, Prog. Solid State Chem., 28, 201 – 281 (2000).
W. Jeitschko, H. Nowotny, and F. Benesovsky, Monatsh. Chen., 94, 672 – 676 (1963).
Y. C. Zhou and Z. M. Sun, Phys. Rev. B, 61, 12570 – 12573 (2000).
Z. B. Ge, K. X. Chen, J. M. Gou, et al., J. Euro Ceram. Soc., 23, 567 – 574 (2003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallovedenie i Termicheskaya Obrabotka Metallov, No. 1, pp. 47 – 50, January, 2011.
Rights and permissions
About this article
Cite this article
Zhu, J., Qi, G., Wang, F. et al. Effect of aluminum content on synthesis of Ti2AlC and Ti3AlC2 during treatment in a high-energy mill and hot pressing. Met Sci Heat Treat 53, 45–48 (2011). https://doi.org/10.1007/s11041-011-9338-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11041-011-9338-6