Abstract
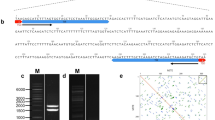
Jatropha curcas is a potential biodiesel crop and a highly adaptable species to various agro-climatic conditions. In this study, we have utilized transposable elements’ (TE) repeat junctions (RJs) which are an important constituent of the genome, used to form a genome-wide molecular marker platform owing to its use in genomic studies of plants. We screened our previously generated Jatropha hybrid genome assembly of size 265 Mbp using RJPrimers pipeline software and identified a total of 1274 TE junctions. For the predicted RJs, we designed 2868 polymerase chain reaction (PCR) based RJ markers (RJMs) flanking the junction regions. In addition to marker design, the identified RJs were utilized to detect 225,517 TEs across the genome. The different types of transposable repeat elements mainly were scattered into Retro, LTR, Copia and Gypsy categories. The efficacy of the designed markers was tested by utilizing a subset of RJMs selected randomly. We have validated 96 randomly selected RJ primers in a group of 32 J. curcas genotypes and more than 90% of the markers effectively intensified as amplicons. Of these, 10 primers were shown to be polymorphic in estimating genetic diversity among the 32 Jatropha lines. UPGMA cluster analysis revealed the formation of two clusters such as A and B exhibiting 85.5% and 87% similarity coefficient respectively. The various RJMs identified in this study could be utilized as a significant asset in Jatropha functional genomics including genome determination, mapping and marker-assisted selection.




Similar content being viewed by others
Abbreviations
- TE:
-
Transposable element
- RJ:
-
Repeat junctions
- LTR:
-
Long terminal repeat
- RJM:
-
Repeat junction marker
- RJJM:
-
Repeat junction–junction marker
- ISBP:
-
Insertion-site-based polymorphism junctions
- RBIP:
-
Retrotransposon based insertion polymorphism
- IRAP:
-
Inter-retrotransposon-amplified polymorphism
References
Sun QB, Li LF, Li Y, Wu GJ, Ge XJ (2008) SSR and AFLP959 markers reveal low genetic diversity in the biofuel plant Jatropha curcas in China. Crop Sci 48:1865–1871
Basha SD, Sujatha M (2007) Inter- and intra-population variability of Jatropha curcas (L.) characterized by RAPD and ISSR markers and development of population specific SCAR markers. Euphytica 156:375–386
Kumar A, Sharma S (2008) An evaluation of multipurpose oil seed crop for industrial uses (Jatropha curcas L.). A review. Ind Crop Prod 28:1–10
Carvalho CR, Clarindo WR, Praça MM, Araújo FS, Carels N (2008) Genome size base composition and karyotype of Jatropha curcas L. an important biofuel plant. Plant Sci 174:613–617
Bourque G, Burns KH, Gehring M, Gorbunova V, Seluanov A, Hammell M, Imbeault M, Izsvák Z, Levin HL, Macfarlan TS, Mager DL, Feschotte C (2018) Ten things you should know about transposable elements. Genome Biol 19(1):199
Sato S, Hirakawa H, Isobe S, Fukai E, Watanabe A, Kato M, Kawashima K, Minami C, Muraki A, Nakazaki N, Takahashi C, Nakayama S, Kishida Y, Kohara M, Yamada M, Tsuruoka H, Sasamoto S, Tabata S, Aizu T, Toyoda A et al (2011) Sequence analysis of the genome of an oil-bearing tree Jatropha curcas L. DNA Res 18(1):65–76. https://doi.org/10.1093/dnares/dsq030
Hirakawa H, Tsuchimoto S, Sakai H, Nakayama S, Fujishiro T, Kishida Y, Kohara M et al (2012) Upgraded genomic information of Jatropha curcas L. Plant Biotechnol 2:123–130
Wu P, Zhou C, Cheng S, Wu Z, Lu W, Han J, Chen Y, Chen Y, Ni P, Wang Y, Xu X, Huang Y, Song C, Wang Z, Shi N, Zhang X, Fang X, Yang Q, Jiang H, Chen Y, Wu G (2015) Integrated genome sequence and linkage map of physic nut (Jatropha curcas L.), a biodiesel plant. Plant J 81(5):810–821. https://doi.org/10.1111/tpj.12761
Ha J, Shim S, Lee T, Kang YJ, Hwang WJ, Jeong H, Laosatit K, Lee J, Kim SK, Satyawan D, Lestari P, Yoon MY, Kim MY, Chitikineni A, Tanya P, Somta P, Srinives P, Varshney RK, Lee SH (2019) Genome sequence of Jatropha curcas L., a non-edible biodiesel plant, provides a resource to improve seed-related traits. Plant Biotechnol J 17(2):517–530. https://doi.org/10.1111/pbi.12995
Alipour A, Tsuchimoto S, Sakai H, Ohmido N, Fukui K (2013) Structural characterization of copia-type retrotransposons leads to insights into the marker development in a biofuel crop Jatropha curcas L. Biotechnol Biofuels 6:129
Mittal N, Dubey AK (2010) A novel set of highly polymorphic chloroplast microsatellite and ISSR markers for the biofuel crop Jatropha curcas. Eur Asia J Biosci 4:119–131
Alipour A, Tsuchimoto S, Fukui K (2017) Molecular markers in Jatropha: current status and future possibilities. In: Tsuchimoto S (ed) The Jatropha genome. Compendium of plant genomes. Springer, Cham
Wanjugi H, Coleman Derr D, Huo N, Kianian SF, Luo MC, Wu J, Anderson O, Gu YQ (2009) Rapid development of PCR-based genome-specific repetitive DNA junction markers in wheat. Genome 52:576–587
Waugh R, McLean K, Flavell AJ, Pearce SR, Kumar A, Thomas BB, Powell W (1997) Genetic distribution of Bare-1-like retro-transposable elements in the barley genome revealed by sequence-specific amplification polymorphisms (S-SAP). Mol Gen Genet 253:687–694
Flavell AJ, Knox MR, Pearce SR, Ellis TH (1998) Retrotransposon-based insertion polymorphisms (RBIP) for high throughput marker analysis. Plant J 16:643–650
Kalendar R, Schulman AH (2006) IRAP and REMAP for retrotransposon-based genotyping and fingerprinting. Nat Protoc 1:2478–2484
Luce AC, Sharma A, Mollere OS, Wolfgruber TK, Nagaki K, Jiang J, Presting GG, Dawe RK (2006) Precise centromere mapping using a combination of repeat junction markers and chromatin immuno precipitation-polymerase chain reaction. Genetics 174:1057–1061
Devos KM, Ma J, Pontaroli AC, Pratt LH, Bennetzen JL (2005) Analysis and mapping of randomly chosen bacterial artificial chromosome clones from hexaploid bread wheat. Proc Natl Acad Sci USA 102:19243–19248
Paux E, Roger D, Badaeva E, Gay G, Bernard M, Sourdille P, Feuillet C (2006) Characterizing the composition and evolution of homoeologous genomes in hexaploid wheat through BAC-end sequencing on chromosome 3B. Plant J 48:463–474
Alipour A, Cartagena JA, Tsuchimoto S et al (2014) Identification and characterization of novel Gypsy-type retrotransposons in a biodiesel crop, Jatropha curcas L. Plant Mol Biol Rep 32:923–930
Li H, Tsuchimoto S, Harada K, Yamasaki M, Sakai H, Wada N, Alipour A, Sasai T, Tsunekawa A, Tsujimoto H, Ando T, Tomemori H, Sato S, Hirakawa H, Quintero VP, Zamarripa A, Santos P, Hegazy A, Ali AM, Fukui K (2017) Genetic tracing of Jatropha curcas L. from its Mesoamerican origin to the world. Front Plant Sci 8:1539. https://doi.org/10.3389/fpls.2017.01539
Li W, Zhang P, Fellers JP, Friebe B, Gill BS (2004) Sequence composition, organization, and evolution of the core Triticeae genome. Plant J 40(4):500–511
Bartos J, Paux E, Kofler R, Havrankova M, Kopecky D, Suchankova P, Safar J, Simkova H, Town CD, Lelley T, Feuillet C, Dolezel J (2008) A first survey of the rye (Secale cereale) genome composition through BAC end sequencing of the short arm of chromosome 1R. BMC Plant Biol 8:95–106
Wicker T, Taudien S, Houben A et al (2009) A whole-genome snapshot of 454 sequences exposes the composition of the barley genome and provides evidence for parallel evolution of genome size in wheat and barley. Plant J 59(5):712–722. https://doi.org/10.1111/j.1365-313X.2009.03911.x
Metzker ML (2010) Sequencing technologies—the next generation. Nat Rev Genet 11(1):31–46. https://doi.org/10.1038/nrg2626
Yepuri V (2016) Molecular mapping of QTLs for economically important traits in sesame (Sesamum indicum L.). Thesis, Jawaharlal Nehru Technological University, Hyderabad
Kancharla N, Jalali S, Narasimham JV, Nair V, Yepuri V, Thakkar B, Reddy V, Kuriakose B, Madan N, Arockiasamy S (2019) De Novo sequencing and hybrid assembly of the biofuel crop Jatropha curcas L. identification of quantitative trait loci for geminivirus resistance. Genes 10:69
You FM, Wanjugi H, Huo N, Lazo GR, Luo M-C, Anderson OD, Dvorak J, Gu YQ (2010) RJPrimers: unique transposable element insertion junction discovery and PCR primer design for marker development. Nucleic Acids Res 38:W313–W320
Spannagl M, Haberer G, Ernst R, Schoof H, Mayer KF (2007) MIPS plant genome information resources. Methods Mol Biol 406:137–159
Ouyang S, Buell CR (2004) The TIGR Plant Repeat Databases: a collective resource for the identification of repetitive sequences in plants. Nucleic Acids Res 32:D360–D363
Hammer O, Harper DAT, Ryan PD (2001) PAST: paleontological statistical software package for education and data analysis. Palaeontol Electron 4(1)
Bennetzen JL (2000) Transposable element contributions to plant gene and genome evolution. Plant Mol Biol 42:251–269
Yadav CB, Bonthala VS, Muthamilarasan M, Pandey G, Khan Y, Prasad M (2015) Genome-wide development of transposable elements-based markers in foxtail millet and construction of an integrated database. DNA Res 22(1):79–90. https://doi.org/10.1093/dnares/dsu039
Zhang G, Liu X, Quan Z et al (2012) Genome sequence of foxtail millet (Setaria italica) provides insights into grass evolution and biofuel potential. Nat Biotechnol 30(6):549–554. https://doi.org/10.1038/nbt.2195
Wicker T, Guyot R, Yahiaoui N, Keller B (2003) CACTA transposons in Triticeae. A diverse family of high-copy repetitive elements. Plant Physiol 132:52–63
Sumner AT, Torre JDL, Stuppia L (1993) The distribution of genes on chromosomes: a cytological approach. J Mol Evol 37:117–122
Pestsova E, Ganal MW, Roder MS (2000) Isolation and mapping of microsatellite markers specific for the D genome of bread wheat. Genome 43:689–697. https://doi.org/10.1139/gen-43-4-689
Qi LL, Echalier B, Chao S, Lazo GR, Butler GE, Anderson OD et al (2004) A chromosome bin map of 16,000 expressed sequence tag loci and distribution of genes among the three genomes of polyploid wheat. Genetics 168:701–712. https://doi.org/10.1534/genetics.104.034868
Yepuri V, Narasimham JV, Sai Shankar P, Kancharla N, Jalali S, Arockiasamy S (2018) Influence of genetic and morphological markers in evaluation of Jatropha diversity. Int J Pure Appl Biosci 6(5):114–123. https://doi.org/10.18782/2320-7051.6780
SanMiguel P, Gaut BS, Tikhonov A, Nakajima Y, Bennetzen JL (1998) The palaeontology of inter-gene retro transposons of maize. Nat Genet 20:43–45
Hua-Van A, Le Rouzic A, Boutin TS, Filée J, Capy P (2011) The struggle for life of the genome’s selfish architects. Biol Direct 6:19. https://doi.org/10.1186/1745-6150-6-19
Poczai P, Varga I, Laos M, Cseh A, Bell N, Valkonen JP, Hyvönen J (2013) Advances in plant gene-targeted and functional markers: a review. Plant Methods 9:6
Mazaheri MP, Kianian MA, Mergoum M et al (2014) Transposable element junctions in marker development and genomic characterization of barley. Plant Genome 7(1):1–8
Acknowledgements
The authors gratefully acknowledged the support and encouragement of the leadership of Dr. Makarand Phadke, Dr. Ajit Sapre and Dr. Santanu Dasgupta at the Reliance Industries Limited in carrying out the research work.
Author information
Authors and Affiliations
Contributions
Conceptualization, VY, SA; Data curation, SJ; Formal analysis, SJ, VY; Supervision, SA; Writing original draft: VY, SJ; Writing: review and editing, VY, SJ, NK and SA; VBR, Contributed in whole genome sequence of Jatropha.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11033_2020_5579_MOESM1_ESM.jpg
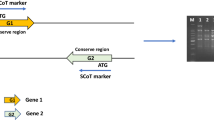
Supplementary file2 (JPG 89 kb) Supplementary Fig. 1 Types of repeat junctions identified in this study. Red colour represents repeat junctions. Orange and green represent different types of TE. Black colour represents unique gene or unknown sequence (adopted from Frank et al. 2010)
11033_2020_5579_MOESM2_ESM.jpg
Supplementary file3 (JPG 175 kb) Supplementary Fig. 2 Primer designing strategies for different TE junctions. Green, orange and pink colour represent different types of transposable element (TE) sequences. Red colour represents repeat junctions. Black colour represents unique gene or unknown sequence. The arrow marked with F1 and F2 are forward primers and R1 and R2 as reverse primer for each type of junctions (Adopted from Frank et al. 2010)
Rights and permissions
About this article
Cite this article
Yepuri, V., Jalali, S., Kancharla, N. et al. Development of genome wide transposable elements based repeat junction markers in Jatropha (Jatropha curcas L.). Mol Biol Rep 47, 5091–5099 (2020). https://doi.org/10.1007/s11033-020-05579-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05579-0