Abstract
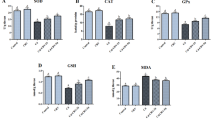
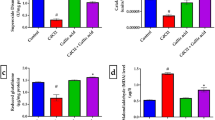
This study aimed to evaluate the potential neuroprotective effect of royal jelly (RJ) against Cd-induced neuronal damage. Twenty-eight adult mice were placed equally into four groups. The control group received intraperitoneal (IP) injections of normal saline; the cadmium chloride (CdCl2) group was IP-injected 6.5 mg/kg (mg per kg of bodyweight) CdCl2; the RJ group was gavaged 85 mg/kg RJ; and the RJ + CdCl2 group was orally administered 85 mg/kg RJ 2 h before receiving IP-injections of 6.5 mg/kg CdCl2. All groups were treated for seven consecutive days and the mice were decapitated 24 h after the final dose. Cd accumulation was recorded in the cortical homogenates, accompanied by elevated levels of lipid peroxidation, nitric oxide, tumor necrosis factor-α, interleukin-1β, and the pro-apoptotic mRNA Bax and caspase-3. Meanwhile, significantly decreased levels of detoxifying antioxidant enzymes including GSH-Px, GSH-R, SOD, and CAT, anti-apoptotic mRNA Bcl-2, and monoamines such as norepinephrine, dopamine, and serotonin were also observed, along with reduced gene expression of Nrf2-dependent antioxidants. Interestingly, in mice pretreated with RJ, the assessed parameters remained near normal levels. Our data provide evidence that RJ treatment has the potential to protect cortical neurons in Cd-intoxicated mice via its antioxidant, anti-inflammatory, anti-apoptotic, and neuromodulatory activity.









Similar content being viewed by others
References
Thevenod F, Lee WK (2013) Cadmium and cellular signaling cascades: interactions between cell death and survival pathways. Arch Toxicol 87(10):1743–1786. https://doi.org/10.1007/s00204-013-1110-9
Bernhoft RA (2013) Cadmium toxicity and treatment. Sci World J 2013:394652. https://doi.org/10.1155/2013/394652
Ercal N, Gurer-Orhan H, Aykin-Burns N (2001) Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem 1(6):529–539
Elkhadragy MF, Kassab RB, Metwally DM, Almeer R, Abdel-Gaber R, Al-Olayan EM, Essawy EA, Amin HK, Abdel Moneim AE (2018) Protective effects of Fragaria ananassa methanolic extract in a rat model of cadmium chloride-induced neurotoxicity. Biosci Rep. https://doi.org/10.1042/bsr20180861
Barnham KJ, Bush AI (2008) Metals in Alzheimer’s and Parkinson’s diseases. Curr Opin Chem Biol 12(2):222–228. https://doi.org/10.1016/j.cbpa.2008.02.019
Ashok A, Rai NK, Tripathi S, Bandyopadhyay S (2015) Exposure to As-, Cd-, and Pb-mixture induces Abeta, amyloidogenic APP processing and cognitive impairments via oxidative stress-dependent neuroinflammation in young rats. Toxicol Sci 143(1):64–80. https://doi.org/10.1093/toxsci/kfu208
Yuan Y, Wang Y, Hu FF, Jiang CY, Zhang YJ, Yang JL, Zhao SW, Gu JH, Liu XZ, Bian JC, Liu ZP (2016) Cadmium activates reactive oxygen species-dependent AKT/mTOR and mitochondrial apoptotic pathways in neuronal cells. Biomed Environ Sci 29(2):117–126. https://doi.org/10.3967/bes2016.013
Al Omairi NE, Radwan OK, Alzahrani YA, Kassab RB (2018) Neuroprotective efficiency of Mangifera indica leaves extract on cadmium-induced cortical damage in rats. Metab Brain Dis. https://doi.org/10.1007/s11011-018-0222-6
Yuan Y, Jiang CY, Xu H, Sun Y, Hu FF, Bian JC, Liu XZ, Gu JH, Liu ZP (2013) Cadmium-induced apoptosis in primary rat cerebral cortical neurons culture is mediated by a calcium signaling pathway. PLoS ONE 8(5):e64330. https://doi.org/10.1371/journal.pone.0064330
Almeer RS, Alarifi S, Alkahtani S, Ibrahim SR, Ali D, Moneim A (2018) The potential hepatoprotective effect of royal jelly against cadmium chloride-induced hepatotoxicity in mice is mediated by suppression of oxidative stress and upregulation of Nrf2 expression. Biomed Pharmacother 106:1490–1498. https://doi.org/10.1016/j.biopha.2018.07.089
McCarty MF (2012) Zinc and multi-mineral supplementation should mitigate the pathogenic impact of cadmium exposure. Med Hypotheses 79(5):642–648. https://doi.org/10.1016/j.mehy.2012.07.043
Fratini F, Cilia G, Mancini S, Felicioli A (2016) Royal Jelly: An ancient remedy with remarkable antibacterial properties. Microbiol Res 192:130–141. https://doi.org/10.1016/j.micres.2016.06.007
Melliou E, Chinou I (2005) Chemistry and bioactivity of royal jelly from Greece. J Agric Food Chem 53(23):8987–8992. https://doi.org/10.1021/jf051550p
Malka O, Karunker I, Yeheskel A, Morin S, Hefetz A (2009) The gene road to royalty–differential expression of hydroxylating genes in the mandibular glands of the honeybee. FEBS J 276(19):5481–5490. https://doi.org/10.1111/j.1742-4658.2009.07232.x
Zhang L, Fang Y, Li R, Feng M, Han B, Zhou T, Li J (2012) Towards posttranslational modification proteome of royal jelly. J Proteom 75(17):5327–5341. https://doi.org/10.1016/j.jprot.2012.06.008
Aslan Z, Aksoy L (2015) Anti-inflammatory effects of royal jelly on ethylene glycol induced renal inflammation in rats. Int Braz J Urol 41(5):1008–1013. https://doi.org/10.1590/S1677-5538.IBJU.2014.0470
Mohamed AA, Galal AA, Elewa YH (2015) Comparative protective effects of royal jelly and cod liver oil against neurotoxic impact of tartrazine on male rat pups brain. Acta Histochem 117(7):649–658. https://doi.org/10.1016/j.acthis.2015.07.002
Yoshida M, Hayashi K, Watadani R, Okano Y, Tanimura K, Kotoh J, Sasaki D, Matsumoto K, Maeda A (2017) Royal jelly improves hyperglycemia in obese/diabetic KK-Ay mice. J Vet Med Sci 79(2):299–307. https://doi.org/10.1292/jvms.16-0458
Zhang S, Shao Q, Geng H, Su S (2017) The effect of royal jelly on the growth of breast cancer in mice. Oncol Lett 14(6):7615–7621. https://doi.org/10.3892/ol.2017.7078
Pyrzanowska J, Wawer A, Joniec-Maciejak I, Piechal A, Blecharz-Klin K, Graikou K, Chinou I, Widy-Tyszkiewicz E (2018) Long-term administration of Greek Royal Jelly decreases GABA concentration in the striatum and hypothalamus of naturally aged Wistar male rats. Neuroscience Lett 675:17–22. https://doi.org/10.1016/j.neulet.2018.03.034
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70(1):158–169
De Vega L, Fernandez RP, Mateo MC, Bustamante JB, Herrero AM, Munguira EB (2002) Glutathione determination and a study of the activity of glutathione-peroxidase, glutathione-transferase, and glutathione-reductase in renal transplants. Ren Fail 24(4):421–432
Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46(2):849–854
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126(1):131–138
Shackelford C, Long G, Wolf J, Okerberg C, Herbert R (2002) Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicol Pathol 30:93–96
Branca JJV, Morucci G, Maresca M, Tenci B, Cascella R, Paternostro F, Ghelardini C, Gulisano M, Di Cesare Mannelli L, Pacini A (2018) Selenium and zinc: two key players against cadmium-induced neuronal toxicity. Toxicol In Vitro 48:159–169. https://doi.org/10.1016/j.tiv.2018.01.007
Goncalves JF, Fiorenza AM, Spanevello RM, Mazzanti CM, Bochi GV, Antes FG, Stefanello N, Rubin MA, Dressler VL, Morsch VM, Schetinger MR (2010) N-acetylcysteine prevents memory deficits, the decrease in acetylcholinesterase activity and oxidative stress in rats exposed to cadmium. Chem Biol Interact 186(1):53–60. https://doi.org/10.1016/j.cbi.2010.04.011
Abdel Moneim AE, Bauomy AA, Diab MM, Shata MT, Al-Olayan EM, El-Khadragy MF (2014) The protective effect of Physalis peruviana L. against cadmium-induced neurotoxicity in rats. Biol Trace Elem Res 160(3):392–399. https://doi.org/10.1007/s12011-014-0066-9
Sultana R, Perluigi M, Allan Butterfield D (2013) Lipid peroxidation triggers neurodegeneration: a redox proteomics view into the Alzheimer disease brain. Free Radic Biol Med 62:157–169. https://doi.org/10.1016/j.freeradbiomed.2012.09.027
Wei T, Chen C, Hou J, Xin W, Mori A (2000) Nitric oxide induces oxidative stress and apoptosis in neuronal cells. Biochim Biophys Acta 1498(1):72–79
Guo H, Ekusa A, Iwai K, Yonekura M, Takahata Y, Morimatsu F (2008) Royal jelly peptides inhibit lipid peroxidation in vitro and in vivo. J Nutr Sci Vitaminol 54(3):191–195
Shagirtha K, Muthumani M, Prabu SM (2011) Melatonin abrogates cadmium induced oxidative stress related neurotoxicity in rats. Eur Rev Med Pharmacol Sci 15(9):1039–1050
Rana SV, Verma S (1996) Protective effects of GSH, vitamin E, and selenium on lipid peroxidation in cadmium-fed rats. Biol Trace Elem Res 51(2):161–168. https://doi.org/10.1007/BF02785435
Amara S, Douki T, Garrel C, Favier A, Ben Rhouma K, Sakly M, Abdelmelek H (2011) Effects of static magnetic field and cadmium on oxidative stress and DNA damage in rat cortex brain and hippocampus. Toxicol Ind Health 27(2):99–106. https://doi.org/10.1177/0748233710381887
Wang B, Du Y (2013) Cadmium and its neurotoxic effects. Oxidative Med Cell Longev 2013:898034. https://doi.org/10.1155/2013/898034
Teixeira RR, de Souza AV, Peixoto LG, Machado HL, Caixeta DC, Vilela DD, Baptista NB, Franci CR, Espindola FS (2017) Royal jelly decreases corticosterone levels and improves the brain antioxidant system in restraint and cold stressed rats. Neurosci Lett 655:179–185. https://doi.org/10.1016/j.neulet.2017.07.010
Aslan A, Cemek M, Buyukokuroglu ME, Altunbas K, Bas O, Yurumez Y (2012) Royal jelly can diminish secondary neuronal damage after experimental spinal cord injury in rabbits. Food Chem Toxicol 50(7):2554–2559. https://doi.org/10.1016/j.fct.2012.04.018
Ahmed MM, El-Shazly SA, Alkafafy ME, Mohamed AA, Mousa AA (2018) Protective potential of royal jelly against cadmium-induced infertility in male rats. Andrologia. https://doi.org/10.1111/and.12996
Moutsatsou P, Papoutsi Z, Kassi E, Heldring N, Zhao C, Tsiapara A, Melliou E, Chrousos GP, Chinou I, Karshikoff A, Nilsson L, Dahlman-Wright K (2010) Fatty acids derived from royal jelly are modulators of estrogen receptor functions. PLoS ONE 5(12):e15594. https://doi.org/10.1371/journal.pone.0015594
Prokai-Tatrai K, Perjesi P, Rivera-Portalatin NM, Simpkins JW, Prokai L (2008) Mechanistic investigations on the antioxidant action of a neuroprotective estrogen derivative. Steroids 73(3):280–288. https://doi.org/10.1016/j.steroids.2007.10.011
Mladenović J, Ognjanović B, Đorđević N, Matić M, Knežević V, Štajn A, Saičić Z (2014) Protective effects of oestradiol against cadmium-induced changes in blood parameters and oxidative damage in rats. Arch Ind Hyg Toxicol 65:37–46. https://doi.org/10.2478/10004-1254-65-2014-2405
Zhao X, Sun G, Zhang J, Strong R, Dash PK, Kan YW, Grotta JC, Aronowski J (2007) Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke 38(12):3280–3286. https://doi.org/10.1161/STROKEAHA.107.486506
Pan H, Wang H, Zhu L, Mao L, Qiao L, Su X (2011) Depletion of Nrf2 enhances inflammation induced by oxyhemoglobin in cultured mice astrocytes. Neurochem Res 36(12):2434–2441. https://doi.org/10.1007/s11064-011-0571-6
Saleh HM, El-Sayed YS, Naser SM, Eltahawy AS, Onoda A, Umezawa M (2017) Efficacy of alpha-lipoic acid against cadmium toxicity on metal ion and oxidative imbalance, and expression of metallothionein and antioxidant genes in rabbit brain. Environ Sci Pollut Res Int 24(31):24593–24601. https://doi.org/10.1007/s11356-017-0158-0
Freitas M, Fernandes E (2011) Zinc, cadmium and nickel increase the activation of NF-kappaB and the release of cytokines from THP-1 monocytic cells. Metallomics 3(11):1238–1243. https://doi.org/10.1039/c1mt00050k
Liu Z, Li P, Zhao D, Tang H, Guo J (2011) Anti-inflammation effects of Cordyceps sinensis mycelium in focal cerebral ischemic injury rats. Inflammation 34(6):639–644. https://doi.org/10.1007/s10753-010-9273-5
You M-M, Chen Y-F, Pan Y-M, Liu Y-C, Tu J, Wang K, Hu F-L (2018) Royal jelly attenuates LPS-induced inflammation in BV-2 microglial cells through modulating NF-κB and p38/JNK signaling pathways. Mediators Inflamm. https://doi.org/10.1155/2018/7834381
Fernandez EL, Gustafson AL, Andersson M, Hellman B, Dencker L (2003) Cadmium-induced changes in apoptotic gene expression levels and DNA damage in mouse embryos are blocked by zinc. Toxicol Sci 76(1):162–170. https://doi.org/10.1093/toxsci/kfg208
Mahdavi S, Khodarahmi P, Roodbari NH (2018) Effects of cadmium on Bcl-2/Bax expression ratio in rat cortex brain and hippocampus. Hum Exp Toxicol 37(3):321–328. https://doi.org/10.1177/0960327117703687
Annunziato L, Amoroso S, Pannaccione A, Cataldi M, Pignataro G, D’Alessio A, Sirabella R, Secondo A, Sibaud L, Di Renzo GF (2003) Apoptosis induced in neuronal cells by oxidative stress: role played by caspases and intracellular calcium ions. Toxicol Lett 139(2–3):125–133
Chen S, Xu Y, Xu B, Guo M, Zhang Z, Liu L, Ma H, Chen Z, Luo Y, Huang S, Chen L (2011) CaMKII is involved in cadmium activation of MAPK and mTOR pathways leading to neuronal cell death. J Neurochem 119(5):1108–1118. https://doi.org/10.1111/j.1471-4159.2011.07493.x
Afifi OK, Embaby AS (2016) Histological study on the protective role of ascorbic acid on cadmium induced cerebral cortical neurotoxicity in adult male albino rats. J Microsc Ultrastruct 4(1):36–45
Carageorgiou H, Tzotzes V, Pantos C, Mourouzis C, Zarros A, Tsakiris S (2004) In vivo and in vitro effects of cadmium on adult rat brain total antioxidant status, acetylcholinesterase, (Na+, K+)-ATPase and Mg2+-ATPase activities: protection by L-cysteine. Basic Clin Pharmacol Toxicol 94(3):112–118
Slotkin TA (2004) Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol 198(2):132–151. https://doi.org/10.1016/j.taap.2003.06.001
Maodaa SN, Allam AA, Ajarem J, Abdel-Maksoud MA, Al-Basher GI, Wang ZY (2016) Effect of parsley (Petroselinum crispum, Apiaceae) juice against cadmium neurotoxicity in albino mice (Mus musculus). Behav Brain Funct 12(1):6. https://doi.org/10.1186/s12993-016-0090-3
Lizarraga LE, Cholanians AB, Phan AV, Herndon JM, Lau SS, Monks TJ (2015) Vesicular monoamine transporter 2 and the acute and long-term response to 3,4-(±)-methylenedioxymethamphetamine. Toxicol Sci 143(1):209–219
Biradar SM, Joshi H, Chheda TK (2012) Neuropharmacological effect of Mangiferin on brain cholinesterase and brain biogenic amines in the management of Alzheimer’s disease. Eur J Pharmacol 683(1–3):140–147. https://doi.org/10.1016/j.ejphar.2012.02.042
Xu B, Chen S, Luo Y, Chen Z, Liu L, Zhou H, Chen W, Shen T, Han X, Chen L, Huang S (2011) Calcium signaling is involved in cadmium-induced neuronal apoptosis via induction of reactive oxygen species and activation of MAPK/mTOR network. PLoS ONE 6(4):e19052. https://doi.org/10.1371/journal.pone.0019052
Hattori N, Nomoto H, Fukumitsu H, Mishima S, Furukawa S (2007) Royal jelly and its unique fatty acid, 10-hydroxy-trans-2-decenoic acid, promote neurogenesis by neural stem/progenitor cells in vitro. Biomed Res 28(5):261–266
Acknowledgements
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the research Group Project No. RGP-180.
Funding
This research was supported by the Deanship of Scientific Research at King Saud University. Research Project No. RGP-180.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Almeer, R.S., Kassab, R.B., AlBasher, G.I. et al. Royal jelly mitigates cadmium-induced neuronal damage in mouse cortex. Mol Biol Rep 46, 119–131 (2019). https://doi.org/10.1007/s11033-018-4451-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-018-4451-x