Abstract
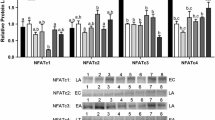
Xenopus laevis, otherwise known as the African clawed frog, undergoes natural dehydration of up to 30% of its total body water during the dry season in sub-Saharan Africa. To survive under these conditions, a variety of physiological and biochemical changes take place in X. laevis. We were interested in understanding the role that the calcineurin-NFAT pathway plays during dehydration stress response in the skeletal muscles of X. laevis. Immunoblotting was performed to characterize the protein levels of NFATc1-4, calcium signalling proteins, in addition to myogenic proteins (MyoD, MyoG, myomaker). In addition, DNA–protein interaction ELISAs were used to assess the binding of NFATs to their consensus binding sequence, and to identify the effect of urea on NFAT-binding. Our results showed that NFATc1 and c4 protein levels decreased during dehydration, and there were no changes in NFATc2, c3, and calcium signalling proteins. However, MyoG and myomaker both showed increases in protein levels during dehydration, thus indicating that the late myogenic program involving myoblast differentiation, but not satellite cell activation and myoblast proliferation, could be involved in preserving the skeletal muscle of X. laevis during dehydration. In addition, we observed that urea seems to reduce NFATc3-binding to DNA during control, but not during dehydration, possibly indicating that NFATc3 is protected from the denaturing effects of urea as it accumulates during dehydration. These findings expand upon our knowledge of adaptive responses to dehydration, and they identify specific protein targets that could be used to protect the skeletal muscle from damage during stress.






Similar content being viewed by others
References
Tinsley RC, Kobel HR (1996) The biology of Xenopus. Zoological Society of London, London, 317–328
Alexander SS, Bellerby CW (1938) Experimental studies on the sexual cycle of the South African clawed toad (Xenopus laevis). J Exp Biol 15:74–81
Romspert AP (1976) Osmoregulation of the african clawed frog. Xenopus laevis, in hypersaline media. Comp Biochem Physiol Part A Physiol 54:207–210. https://doi.org/10.1016/S0300-9629(76)80098-9
Grundy JE, Storey KB (1994) Urea and salt effects on enzymes from estivating and non-estivating amphibians. Mol Cell Biochem 131:9–17. https://doi.org/10.1007/BF01075719
Balinsky JB, Choritz EL, Coe CGL, van der Schans GS (1967) Amino acid metabolism and urea synthesis in naturally aestivating Xenopus laevis. Comp Biochem Physiol 22:59–68. https://doi.org/10.1016/0010-406X(67)90166-1
Balinsky JB, Cragg MM, Baldwin E (1961) The adaptation of amphibian waste nitrogen excretion to dehydration. Comp Biochem Physiol 3:236–244. https://doi.org/10.1016/0010-406X(61)90009-3
Janssens PA (1964) Urea production and transaminase activity in Xenopus laevis Daudin. Comp Biochem Physiol 13:217–224. https://doi.org/10.1016/0010-406X(64)90118-5
Seiter P, Schultheiss H, Hanke W (1978) Osmotic stress and excretion of ammonia and urea in Xenopus laevis. Comp Biochem Physiol Part A Physiol 61:571–576. https://doi.org/10.1016/0300-9629(78)90130-5
Malik AI, Storey KB (2009) Activation of antioxidant defense during dehydration stress in the African clawed frog. Gene 442:99–107. https://doi.org/10.1016/j.gene.2009.04.007
Malik AI, Storey KB (2009) Activation of extracellular signal-regulated kinases during dehydration in the African clawed frog, Xenopus laevis. J Exp Biol 212:2595–2603. https://doi.org/10.1242/jeb.030627
Malik AI, Storey KB (2011) Transcriptional regulation of antioxidant enzymes by FoxO1 under dehydration stress. Gene 485:114–119. https://doi.org/10.1016/j.gene.2011.06.014
Flanagan WM, Corthésy B, Bram RJ, Crabtree GR (1991) Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature 352:803–807
Clipstone NA, Crabtree GR (1992) Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature 355:717–719. https://doi.org/10.1038/357695a0
Lin H, Sue YM, Chou Y et al (2010) Activation of a nuclear factor of activated T-lymphocyte-3 (NFAT3) by oxidative stress in carboplatin-mediated renal apoptosis. Br J Pharmacol 161:1661–1676. https://doi.org/10.1111/j.1476-5381.2010.00989.x
Arron JR, Winslow MM, Polleri A et al (2006) NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature 441:595–600. https://doi.org/10.1038/nature04678
Chang CP, Neilson JR, Bayle JH et al (2004) A field of myocardial-endocardial NFAT signaling underlies heart valve morphogenesis. Cell 118:649–663. https://doi.org/10.1016/j.cell.2004.08.010
Graef IA, Chen F, Chen L et al (2001) Signals transduced by Ca2+/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell 105:863–875. https://doi.org/10.1016/S0092-8674(01)00396-8
Zhang Y, Storey KB (2015) Expression of nuclear factor of activated T cells (NFAT) and downstream muscle-specific proteins in ground squirrel skeletal and heart muscle during hibernation. Mol Cell Biochem 412:27–40. https://doi.org/10.1007/s11010-015-2605-x
Armand A-S, Bourajjaj M, Martínez-Martínez S et al (2008) Cooperative synergy between NFAT and MyoD regulates myogenin expression and myogenesis. J Biol Chem 283:29004–29010. https://doi.org/10.1074/jbc.M801297200
Nicolas N, Gallien CL, Chanoine C (1996) Analysis of MyoD, myogenin, and muscle-specific gene mRNAs in regenerating Xenopus skeletal muscle. Dev Dyn 207:60–68. doi: 10.1002/(SICI)1097-0177(199609)207:1<100::AID-AJA9>3.0.CO;2-M
Boudjelida H, Muntz L (1987) Multinucleation during myogenesis of the myotome of Xenopus laevis: a qualitative study. Development 101:583–590
Daughters RS, Chen Y, Slack JMW (2011) Origin of muscle satellite cells in the Xenopus embryo. Development 138:821–830. https://doi.org/10.1242/dev.056481
Hudson NJ, Franklin CE (2002) Effect of aestivation on muscle characteristics and locomotor performance in the Green-striped burrowing frog, Cyclorana alboguttata. J Comp Physiol B Biochem Syst Environ Physiol 172:177–182. https://doi.org/10.1007/s00360-001-0242-z
Mantle BL, Hudson NJ, Harper GS et al (2009) Skeletal muscle atrophy occurs slowly and selectively during prolonged aestivation in Cyclorana alboguttata (Gunther 1867). J Exp Biol 212:3664–3672. https://doi.org/10.1242/jeb.033688
Zhang Y, Storey KB (2016) Regulation of gene expression by NFAT transcription factors in hibernating ground squirrels is dependent on the cellular environment. Cell Stress Chaperones 21:883–894. https://doi.org/10.1007/s12192-016-0713-5
Millay DP, O’Rourke JR, Sutherland LB et al (2013) Myomaker is a membrane activator of myoblast fusion and muscle formation. Nature 499:301–305. https://doi.org/10.1038/nature12343
Rusnak F, Mertz P (2000) Calcineurin: form and function. Physiol Rev 80:1483–1521. https://doi.org/10.1172/JCI57909.date
Kretsinger R (1987) Calcium coordination and the calmodulin fold: divergent versus convergent evolution. Cold Spring Harb Symp Quant Biol 52:499–510
Yang S, Klee CB (2000) Low affinity Ca2+-binding sites of calcineurin B mediate conformational changes in calcineurin A. Biochemistry 39:16147–16154
Klee CB, Crouch TH, Krinks MH (1979) Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci 76:6270–6273. https://doi.org/10.1073/pnas.76.12.6270
Burkard N (2005) Targeted proteolysis sustains calcineurin activation. Circulation 111:1045–1053. https://doi.org/10.1161/01.CIR.0000156458.80515.F7
Lee SH, Choi J, Kim H et al (2014) FK506 reduces calpain-regulated calcineurin activity in both the cytoplasm and the nucleus. Anat Cell Biol 47:91–100. https://doi.org/10.5115/acb.2014.47.2.91
Shioda N, Moriguchi S, Shirasaki Y, Fukunaga K (2006) Generation of constitutively active calcineurin by calpain contributes to delayed neuronal death following mouse brain ischemia. J Neurochem 98:310–320. https://doi.org/10.1111/j.1471-4159.2006.03874.x
Wray S, Wilkie DR (1995) The relationship between plasma urea levels and some muscle trimethylamine levels in Xenopus laevis: a 31P and 14N nuclear magnetic resonance study. J Exp Biol 198:373–378
Eaton SL, Roche SL, Llavero Hurtado M et al (2013) Total protein analysis as a reliable loading control for quantitative fluorescent western blotting. PLoS ONE 8:e72457. https://doi.org/10.1371/journal.pone.0072457
Hung H-F, Wang B-W, Chang H, Shyu K-G (2008) The molecular regulation of resistin expression in cultured vascular smooth muscle cells under hypoxia. J Hypertens 26:2349–2360. https://doi.org/10.1097/HJH.0b013e328311fa30
Rao A, Luo C, Hogan PG (1997) Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol 15:707–747. https://doi.org/10.1146/annurev.immunol.15.1.707
Demonbreun AR, Lapidos KA, Heretis K et al (2010) Myoferlin regulation by NFAT in muscle injury, regeneration and repair. J Cell Sci 123:2413–2422. https://doi.org/10.1242/jcs.065375
Cotton CJJ, Harlow HJJ (2010) Avoidance of skeletal muscle atrophy in spontaneous and facultative hibernators. Physiol Biochem Zool 83:551–560. https://doi.org/10.1086/650471
Xu R, Andres-Mateos E, Mejias R et al (2013) Hibernating squirrel muscle activates the endurance exercise pathway despite prolonged immobilization. Exp Neurol 247:392–401. https://doi.org/10.1016/j.expneurol.2013.01.005
Cotton CJ (2016) Skeletal muscle mass and composition during mammalian hibernation. J Exp Biol 219:226–234. https://doi.org/10.1242/jeb.125401
Brooks NE, Myburgh KH, Storey KB (2015) Muscle satellite cells increase during hibernation in ground squirrels. Comp Biochem Physiol Part B 189:55–61. https://doi.org/10.1016/j.cbpb.2015.07.006
Tessier SN, Storey KB (2010) Expression of myocyte enhancer factor-2 and downstream genes in ground squirrel skeletal muscle during hibernation. Mol Cell Biochem 344:151–162. https://doi.org/10.1007/s11010-010-0538-y
Nicolas N, Mira J-C, Gallien CL, Chanoine C (1998) Localization of Myf-5, MRF4 and a cardiac actin mRNAs in regenerating Xenopus skeletal muscle. Académie des Sci 355–364
Hirotani H, Tuohy NA, Woo J-T et al (2004) The calcineurin/nuclear factor of activated T cells signaling pathway regulates osteoclastogenesis in RAW264.7 cells. J Biol Chem 279:13984–13992. https://doi.org/10.1074/jbc.M213067200
Li Q, Lin X, Yang X, Chang J (2010) NFATc4 is negatively regulated in miR-133a-mediated cardiomyocyte hypertrophic repression. Am J Physiol Heart Circ Physiol 298:H1340–H1347. https://doi.org/10.1152/ajpheart.00592.2009
Li M, Wang N, Zhang J et al (2016) MicroRNA-29a-3p attenuates ET-1-induced hypertrophic responses in H9c2 cardiomyocytes. Gene 585:44–50. https://doi.org/10.1016/j.gene.2016.03.015
Wu CW, Biggar KK, Storey KB (2013) Dehydration mediated microRNA response in the African clawed frog Xenopus laevis. Gene 529:269–275. https://doi.org/10.1016/j.gene.2013.07.064
Bentzinger CF, Wang YX, Rudnicki MA (2012) Building muscle: molecular regulation of myogenesis. Cold Spring Harb Perspect Biol 4:1–16. https://doi.org/10.1101/cshperspect.a008342
Cohen TJ, Waddell DS, Barrientos T et al (2007) The histone deacetylase HDAC4 connects neural activity to muscle transcriptional reprogramming. J Biol Chem 282:33752–33759. https://doi.org/10.1074/jbc.M706268200
Tang H, Macpherson P, Marvin M et al (2009) A histone deacetylase 4/myogenin positive feedback loop coordinates denervation-dependent gene induction and suppression. Mol Biol Cell 20:1120–1131. https://doi.org/10.1091/mbc.E08-07-0759
Acknowledgements
This work was supported by a Discovery grant from the Natural Sciences and Engineering Research Council (NSERC) of Canada (#6793) to Kenneth B. Storey. Kenneth B. Storey holds the Canada Research Chair in Molecular Physiology; Yichi Zhang held a postgraduate Queen Elizabeth II Graduate Scholarship in Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that we have no conflict of interest.
Ethical approval
Animals were cared for in accordance with the guidelines of the Canadian Council on Animal Care and all experimental procedures had the prior approval of the Carleton University Animal Care Committee (protocol #13683).
Rights and permissions
About this article
Cite this article
Zhang, Y., English, S.G. & Storey, K.B. Regulation of nuclear factor of activated T cells (NFAT) and downstream myogenic proteins during dehydration in the African clawed frog. Mol Biol Rep 45, 751–761 (2018). https://doi.org/10.1007/s11033-018-4214-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-018-4214-8