Abstract
This meta-analysis was performed to assess the relationships between the PON1 Q192R (rs662 T>C) polymorphism and the clinical outcome of antiplatelet treatment after percutaneous coronary intervention (PCI). A range of electronic databases were searched: Web of Science (1945–2013), the Cochrane Library Database (Issue 12, 2013), PubMed (1966–2013), EMBASE (1980–2013), CINAHL (1982–2013) and the Chinese Biomedical Database (CBM) (1982–2013) without language restrictions. Meta-analysis was conducted using the STATA 12.0 software. The crude odds ratio (OR) with their 95 % confidence interval (CI) were calculated. Six clinical cohort studies with a total number of 5,189 patients undergoing PCI for coronary heart disease were included. Our meta-analysis revealed that the PON1 Q192R polymorphism was correlated with an increased risk of major adverse cardiovascular events (MACE) in patients receiving antiplatelet treatment after PCI (C allele vs. T allele: OR = 1.22, 95 % CI 1.04–1.43, P = 0.014; CT+CC vs. TT: OR = 1.38, 95 % CI 1.03–1.86, P = 0.029; CC vs. TT: OR = 1.45, 95 % CI 1.05–1.99, P = 0.024; respectively), especially among Asians. Furthermore, we found significantly positive correlations between the PON1 Q192R polymorphism and the incidence of stent thrombosis in patients receiving antiplatelet treatment after PCI (C allele vs. T allele: OR = 1.42, 95 % CI 1.08–1.87, P = 0.011; CT+CC vs. TT: OR = 1.93, 95 % CI 1.01–3.67, P = 0.046; CC vs. TT: OR = 2.18, 95 % CI 1.09–4.35, P = 0.027; respectively). Our meta-analysis of clinical cohort studies provides evidence that the PON1 Q192R polymorphism may increase the risk of MACE and stent thrombosis in patients receiving antiplatelet treatment after PCI.
Similar content being viewed by others
Introduction
Percutaneous coronary intervention (PCI) with coronary stenting has been used in clinical practice for nearly 30 years and has become the most common revascularization treatment for patients with coronary heart disease (CHD) [1]. However, due to the platelet activation induced by factors such as coronary vascular intimal injury and vascular stent implantation in the process of interventional treatment, the risk of thrombosis increases significantly [2]. Therefore, antiplatelet therapy plays a pivotal role in fields of basic science and clinical cardiology [3]. Current guidelines recommend dual antiplatelet therapy that includes aspirin and clopidogrel for at least 12 months after PCI [4]. Despite treatment with antiplatelet therapy, thromboembolic events still occurred in some patients. Recent studies have suggested that platelet-related genetic polymorphisms can modulate the response to these agents [5] and affect clinical outcome in patients receiving antiplatelet therapy after PCI [6].
Paraoxonase 1 (PON1), synthesized mainly in the liver, is a calcium-dependent, multifunctional antioxidant enzyme that is widely distributed and was originally named for its ability to hydrolyze exogenous organophosphates [7]. PON1 is notably involved in detoxifying organophosphate nerve agents [8]. Furthermore, it is transported with high-density lipoprotein in the plasma and functions as an antioxidant, decreasing the production of atherogenic oxidized low-density lipoprotein [9]. The human PON1 gene belongs to the family of serum paraoxonases consisting of PON1–3 that are all located next to each other on the long arm of chromosome 7 (7q21.3–22.1), which spans ~26.2, 30.2 and 36.5 kb, respectively, and consists of nine exons and eight introns [10]. Importantly, PON1 is identified as a key factor in the bioactivation and clinical activity of clopidogrel [11]. In general, dual antiplatelet therapy with clopidogrel and aspirin inhibits platelet function, thereby preventing recurrent cardiovascular events in patients with CHD who are undergoing PCI [12]. Clinically, clopidogrel is a thienopyridine prodrug which must be metabolized by the liver enzyme to generate an active metabolite [13, 14]. Therefore, it has been hypothesized that those common polymorphisms in the PON1 gene may be functional and associated with the antiplatelet therapy after PCI [7]. Recently, several studies have also indicated that PON1 is a major determinant of clopidogrel efficacy [15]. Thus, PON1 genetic polymorphisms might be associated with clinical outcomes and may serve as novel genetic risk factors for adverse events after PCI [6, 11]. However, other studies have suggested that PON1 Q192R (rs662 T>C) did not have any influence on the antiplatelet effect of clopidogrel, either in the acute or chronic phase of therapy after PCI [16, 17]. In view of these conflicting results, we performed a meta-analysis of all available data to evaluate the associations between the PON1 Q192R (rs662 T>C) polymorphism and the clinical outcome of patients receiving antiplatelet therapy after PCI.
Materials and methods
Literature search
The following electronic databases were searched for relevant articles without language restrictions: Web of Science (1945–2013), the Cochrane Library Database (Issue 12, 2013), PubMed (1966–2013), EMBASE (1980–2013), CINAHL (1982–2013) and the Chinese Biomedical Database (CBM) (1982–2013). We used the following keywords and MeSH terms in conjunction with a highly sensitive search strategy: (“percutaneous coronary intervention” or “percutaneous coronary intervention” or “percutaneous coronary revascularization” or “PCI”) and (“antiplatelet therapy” or “triple antiplatelet therapy” or “TAT” or “dual antiplatelet therapy” or “DAT” or “aspirin” or “clopidogrel” or “cilostazol”) and (“aryldialkylphosphatase” or “PON1 protein, human” or “paraoxonase” or “PON1”) and (“genetic polymorphism” or “single nucleotide polymorphism” or “polymorphism” or “SNP” or “mutation” or “variation” or “variant”). We also conducted a manual search to find other potential articles based on references identified in the retrieved articles.
Selection criteria
The following criteria were used to determine eligibility for including studies: (1) the study must be a clinical cohort study; (2) the study must address the relationship between the PON1 Q192R (rs662 T>C) polymorphism and the clinical outcome of patients receiving antiplatelet treatment after PCI; (3) all patients must have conformed to the diagnostic criteria of CHD; (4) the study must provide sufficient information about the frequencies of the PON1 Q192R polymorphism. Article that did not meet the inclusion criteria were excluded. When authors published several studies using the same subjects, either the most recent or largest sample size publication was included.
Data extraction
Two authors used a standardized form to extract the following data from included studies: surname of first author, year of the publication, source of publication, country of origin, ethnicity, language of publication, study type, total number of subjects or samples, source of subjects’ DNA samples, detection method, the incidence of major adverse cardiovascular event (MACE) and stent thrombosis, etc. In cases of conflicting evaluations, disagreements were resolved through discussion and careful reexamination of the full text by the authors.
Methodological assessment
Methodological quality was evaluated separately by two authors using the Newcastle–Ottawa Scale (NOS) criteria [18]. The NOS criteria is based on three aspects: (1) subject selection: 0–4; (2) comparability of subject: 0–2; (3) clinical outcome: 0–3. NOS scores range from 0 to 9 with scores ≥7 indicating good quality.
Statistical analysis
Meta-analysis was performed using the STATA statistical software (version 12.0, Stata Corporation, College Station, TX, USA). Odds ratios (OR) and 95 % confidence intervals (95 % CI) were calculated. The Z test was used to estimate the statistical significance of the pooled ORs. Heterogeneity among studies was estimated with the Cochran’s Q-statistic and I 2 tests [19]. If the Q-test showed a P < 0.05 or I 2 test exhibited >50 %, which indicate significant heterogeneity, the random-effects model was utilized. Otherwise the fixed-effects model was used. We also explored potential sources of heterogeneity using subgroup analyses and meta-regression analyses. In order to evaluate the influence of single studies on the overall estimate, a sensitivity analysis was performed. Funnel plots and Egger’s linear regression test were applied to investigate publication bias [20].
Results
Study selection and characteristics of included studies
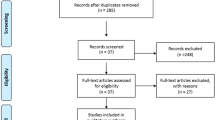
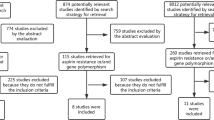
Initially, our search strategy identified 329 articles. We reviewed the titles and abstracts of all articles and excluded 173 articles. After systematically reviewing the remaining full texts, we excluded another 148 articles. In addition, two studies were excluded due to lack of data integrity (Fig. 1). Finally, six clinical cohort studies met our inclusion criteria for qualitative data analysis, with a total of 5,198 CHD patients that underwent PCI with coronary stenting [6, 17, 21–24]. The publication years of eligible studies range from 2011 to 2013. Figure 2 shows the distribution of the number of topic-related literature in electronic databases over the last decade. Overall, three studies were conducted among Asians and three studies among Caucasians. Genotyping was performed using the polymerase chain reaction-ligation detection reaction (PCR–LDR) and TaqMan assay methods. The NOS scores of all included studies were ≥5. We summarized the study characteristics and methodological quality in Table 1.
Quantitative data synthesis
A summary of the findings of this meta-analysis on the correlations between the PON1 Q192R (rs662 T>C) polymorphism and the clinical outcome of antiplatelet treatment after PCI are shown in Table 2. These results indicate that the PON1 Q192R polymorphism was correlated with an increased risk for MACE in patients receiving antiplatelet treatment after PCI (C allele vs. T allele: OR = 1.22, 95 % CI 1.04–1.43, P = 0.014; CT+CC vs. TT: OR = 1.38, 95 % CI 1.03–1.86, P = 0.029; CC vs. TT: OR = 1.45, 95 % CI 1.05–1.99, P = 0.024; respectively) (Fig. 3). Furthermore, we found significant positive correlations between the PON1 Q192R polymorphism and the incidence of stent thrombosis in patients receiving antiplatelet treatment after PCI (C allele vs. T allele: OR = 1.42, 95 % CI 1.08–1.87, P = 0.011; CT+CC vs. TT: OR = 1.93, 95 % CI 1.01–3.67, P = 0.046; CC vs. TT: OR = 2.18, 95 % CI 1.09–4.35, P = 0.027; respectively) (Fig. 4).
Subgroup analyses by ethnicity and sample size demonstrated that the PON1 Q192R polymorphism was correlated with an increased risk of MACE and stent thrombosis in patients receiving antiplatelet treatment after PCI among Asians and in the large sample-size subgroup, but not among Caucasians or in the small sample-size subgroup (as shown in Table 2). Meta-regression analyses results confirmed that none of the considered factors could explain the source of heterogeneity (as shown in Table 3). A sensitivity analysis suggested that no single study significantly influenced the pooled ORs (Fig. 5). Funnel plots demonstrated no evidence of obvious asymmetry (Fig. 6). Egger’s test also did not display strong statistical evidence of publication bias (MACE: t = −2.26, P = 0.109; stent thrombosis: t = 1.01, P = 0.420; respectively).
Discussion
PON1, as an esterase synthesized in the liver with antioxidative and anti-atherogenic properties, is a major anti-atherosclerotic component of high-density lipoproteins (HDL) in blood [11, 25]. The main biologic function of PON1, which is genetically controlled, is determined by its ability to affect the catalytic capability of the enzyme and mediate the rate of the formation of the thiol active metabolite from clopidogrel, which is associated with coronary artery disease [26, 27]. A recently published study suggests that the PON1 genetic polymorphism may play a crucial role in the rate of active metabolite formation from clopidogrel, which in turn affects platelet reactivity and, consequently, the incidence of ischemic events in patients on antiplatelet therapy [7, 27]. A dual antiplatelet therapy of aspirin and clopidogrel can significantly improve the prognosis of major cardiovascular events [28]. Therefore, it is biologically plausible that genetic variations of the PON1 gene may modulate the efficacy of antiplatelet therapy [6].
Our results indicated that the PON1 Q192R polymorphism was strongly correlated with the risk of MACE in patients receiving antiplatelet treatment after PCI. Moreover, we also observed a positive association between the PON1 Q192R polymorphism and the development of stent thrombosis in patients undergoing antiplatelet treatment after PCI, which suggests that the PON1 Q192R polymorphism may affect clinical outcomes in patients receiving antiplatelet treatment after PCI. Although the exact mechanisms by which the PON1 Q192R polymorphism leads to the occurrence of stent thrombosis remain poorly understood, a potential explanation may be that the PON1 Q192R polymorphism has an impact on PON enzyme activity, which is considered to be a key step in clopidogrel bioactivation. It is well known that the response to clopidogrel plays a crucial role in antiplatelet therapy after PCI and that low platelet responsiveness to clopidogrel may lead to a high incidence of atherothrombotic events, including stent thrombosis, the most serious and often fatal clinical event. Thus, the PON1 Q192R polymorphism might be strongly related to clopidogrel metabolism and the development of stent thrombosis in antiplatelet treatment after PCI [6, 11]. Subgroup analysis by ethnicity demonstrated that the PON1 Q192R polymorphism was correlated with the clinical outcomes of antiplatelet therapy after PCI among Asians but not among Caucasians, revealing that there are ethnic differences in the effects of the PON1 Q192R polymorphism on the clinical outcomes of patients receiving antiplatelet treatment after PCI. In short, our findings are consistent with previous studies that the PON1 Q192R polymorphism may contribute to the occurrence of MACE and stent thrombosis, which indicates that the PON1 Q192R polymorphism may be utilized to predict clinical outcome in patients receiving antiplatelet treatment after PCI.
Nevertheless, this meta-analysis also has some limitations. First, our results may not provide sufficient statistical power to estimate the correlations between the PON1 Q192R polymorphism and clinical outcome in patients receiving antiplatelet treatment after PCI due to a relatively small sample size. Second, meta-analysis is a retrospective study that may lead to subject selection bias, and thus the reliability of our results may have been influenced by such a bias. Third, since meta-analysis failed to obtain original data from the included studies, it was limited from further evaluating the potential impact of the PON1 Q192R polymorphism on the clinical outcomes of patients receiving antiplatelet treatment after PCI. Importantly, the inclusion criteria of cases and controls were not well defined in all included studies, which might have also influence our results.
In conclusion, our meta-analysis of clinical cohort studies provides evidence that the PON1 Q192R polymorphism may increase the risk of MACE and stent thrombosis in patients receiving antiplatelet treatment after PCI. Thus, the PON1 Q192R polymorphism may be utilized in predicting the clinical outcome of patients receiving antiplatelet treatment after PCI. However, due to the limitations mentioned above, further detailed studies with larger sample sizes are still needed to provide a more representative statistical analysis.
References
Caggegi A, Capodanno D, Capranzano P, Chisari A, Ministeri M, Mangiameli A, Ronsivalle G, Ricca G et al (2011) Comparison of one-year outcomes of percutaneous coronary intervention versus coronary artery bypass grafting in patients with unprotected left main coronary artery disease and acute coronary syndromes (from the customize registry). Am J Cardiol 108:355–359. doi:10.1016/j.amjcard.2011.03.050
Mause SF, Ritzel E, Liehn EA, Hristov M, Bidzhekov K, Muller-Newen G, Soehnlein O, Weber C (2010) Platelet microparticles enhance the vasoregenerative potential of angiogenic early outgrowth cells after vascular injury. Circulation 122:495–506. doi:10.1161/CIRCULATIONAHA.109.909473
Lhermusier T, Van Rothem J, Garcia C, Gratacap MP, Payrastre B (2011) Targeted drug therapy: the platelet side. Platelets 22:479–484. doi:10.3109/09537104.2011.567423
Chhatriwalla AK, Bhatt DL (2008) Should dual antiplatelet therapy after drug-eluting stents be continued for more than one-year? Dual antiplatelet therapy after drug-eluting stents should be continued for more than one-year and preferably indefinitely. Circ Cardiovasc Interv 1:217–225. doi:10.1161/CIRCINTERVENTIONS.108.811380
Tousoulis D, Paroutoglou IP, Papageorgiou N, Charakida M, Stefanadis C (2010) Recent therapeutic approaches to platelet activation in coronary artery disease. Pharmacol Ther 127:108–120. doi:10.1016/j.pharmthera.2010.05.001
Park KW, Park JJ, Kang J, Jeon KH, Kang SH, Han JK, Lee SE, Yang HM et al (2013) Paraoxonase 1 gene polymorphism does not affect clopidogrel response variability but is associated with clinical outcome after PCI. PLoS One 8:e52779. doi:10.1371/journal.pone.0052779
Trenk D, Hochholzer W, Fromm MF, Zolk O, Valina CM, Stratz C, Neumann FJ (2011) Paraoxonase-1 Q192R polymorphism and antiplatelet effects of clopidogrel in patients undergoing elective coronary stent placement. Circ Cardiovasc Genet 4:429–436. doi:10.1161/CIRCGENETICS.111.960112
Li WF, Costa LG, Richter RJ, Hagen T, Shih DM, Tward A, Lusis AJ, Furlong CE (2000) Catalytic efficiency determines the in vivo efficacy of pon1 for detoxifying organophosphorus compounds. Pharmacogenetics 10:767–779
Hulot JS, Collet JP, Cayla G, Silvain J, Allanic F, Bellemain-Appaix A, Scott SA, Montalescot G (2011) CYP2C19 but not PON1 genetic variants influence clopidogrel pharmacokinetics, pharmacodynamics, and clinical efficacy in post-myocardial infarction patients. Circ Cardiovasc Interv 4:422–428. doi:10.1161/CIRCINTERVENTIONS.111.963025
Wang X, Fan Z, Huang J, Su S, Yu Q, Zhao J, Hui R, Yao Z et al (2003) Extensive association analysis between polymorphisms of PON gene cluster with coronary heart disease in Chinese Han population. Arterioscler Thromb Vasc Biol 23:328–334. doi:10.1161/01.ATV.0000051702.38086.C1
Bouman HJ, Schomig E, van Werkum JW, Velder J, Hackeng CM, Hirschhauser C, Waldmann C, Schmalz HG et al (2011) Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nat Med 17:110–116. doi:10.1038/nm.2281
Lewis JP, Fisch AS, Ryan K, O’Connell JR, Gibson Q, Mitchell BD, Shen H, Tanner K et al (2011) Paraoxonase 1 (PON1) gene variants are not associated with clopidogrel response. Clin Pharmacol Ther 90:568–574. doi:10.1038/clpt.2011.194
Plosker GL, Lyseng-Williamson KA (2007) Clopidogrel: a review of its use in the prevention of thrombosis. Drugs 67:613–646
Pereillo JM, Maftouh M, Andrieu A, Uzabiaga MF, Fedeli O, Savi P, Pascal M, Herbert JM et al (2002) Structure and stereochemistry of the active metabolite of clopidogrel. Drug Metab Dispos 30:1288–1295. doi:10.1124/dmd.30.11.1288
Camps J, Joven J, Mackness B, Mackness M, Tawfik D, Draganov D, Costa LG, Paragh G et al (2011) Paraoxonase-1 and clopidogrel efficacy. Nat Med 17:1041–1042. doi:10.1038/nm.2386
Price MJ, Murray SS, Angiolillo DJ, Lillie E, Smith EN, Tisch RL, Schork NJ, Teirstein PS et al (2012) Influence of genetic polymorphisms on the effect of high- and standard-dose clopidogrel after percutaneous coronary intervention: the gift (genotype information and functional testing) study. J Am Coll Cardiol 59:1928–1937. doi:10.1016/j.jacc.2011.11.068
Chen DY, Wang CY, Wen MS, Lee TH, Chu Y, Hsieh MJ, Chang SH, Lee CH et al (2012) Paraoxonase-1 is not a major determinant of stent thrombosis in a taiwanese population. PLoS One 7:e39178. doi:10.1371/journal.pone.0039178
Stang A (2010) Critical evaluation of the Newcastle–Ottawa Scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605. doi:10.1007/s10654-010-9491-z
Zintzaras E, Ioannidis JP (2005) Hegesma: genome search meta-analysis and heterogeneity testing. Bioinformatics 21:3672–3673. doi:10.1093/bioinformatics/bti536
Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L (2006) Comparison of two methods to detect publication bias in meta-analysis. JAMA 295:676–680. doi:10.1001/jama.295.6.676
Campo G, Ferraresi P, Marchesini J, Bernardi F, Valgimigli M (2011) Relationship between paraoxonase q192r gene polymorphism and on-clopidogrel platelet reactivity over time in patients treated with percutaneous coronary intervention. J Thromb Haemost 9:2106–2108. doi:10.1111/j.1538-7836.2011.04457.x
Sibbing D, Koch W, Massberg S, Byrne RA, Mehilli J, Schulz S, Mayer K, Bernlochner I et al (2011) No association of paraoxonase-1 Q192R genotypes with platelet response to clopidogrel and risk of stent thrombosis after coronary stenting. Eur Heart J 32:1605–1613. doi:10.1093/eurheartj/ehr155
Tang XF, Wang J, Zhang JH, Meng XM, Xu B, Qiao SB, Wu YJ, Chen J et al (2013) Effect of the CYP2C19 2 and 3 genotypes, abcb1 C3435T and pon1 Q192R alleles on the pharmacodynamics and adverse clinical events of clopidogrel in Chinese people after percutaneous coronary intervention. Eur J Clin Pharmacol 69:1103–1112. doi:10.1007/s00228-012-1446-8
Verschuren JJ, Boden H, Wessels JA, van der Hoeven BL, Trompet S, Heijmans BT, Putter H, Guchelaar HJ et al (2013) Value of platelet pharmacogenetics in common clinical practice of patients with st-segment elevation myocardial infarction. Int J Cardiol 167:2882–2888. doi:10.1016/j.ijcard.2012.07.020
Getz GS, Reardon CA (2004) Paraoxonase, a cardioprotective enzyme: continuing issues. Curr Opin Lipidol 15:261–267
Costa LG, Cole TB, Vitalone A, Furlong CE (2005) Measurement of paraoxonase (PON1) status as a potential biomarker of susceptibility to organophosphate toxicity. Clin Chim Acta 352:37–47. doi:10.1016/j.cccn.2004.09.019
Simon T, Steg PG, Becquemont L, Verstuyft C, Kotti S, Schiele F, Ferrari E, Drouet E et al (2011) Effect of paraoxonase-1 polymorphism on clinical outcomes in patients treated with clopidogrel after an acute myocardial infarction. Clin Pharmacol Ther 90:561–567. doi:10.1038/clpt.2011.193
Steinhubl SR, Berger PB, Mann JT III, Fry ET, DeLago A, Wilmer C, Topol EJ (2002) Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 288:2411–2420. doi:10.1001/jama.288.19.2411
Acknowledgments
We would like to acknowledge the reviewers for their helpful comments on this paper. This work was supported by Natural Science Foundation of China (NSFC) (grant #81202598), Shanghai Municipal Public Health Bureau (grant #2009068).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
The Publisher and Editor retract this article in accordance with the recommendations of the Committee on Publication Ethics (COPE). After a thorough investigation we have strong reason to believe that the peer review process was compromised.
About this article
Cite this article
Li, P., Bu, SH., Lu, XT. et al. RETRACTED ARTICLE: Relationships between PON1 Q192R polymorphism and clinical outcome of antiplatelet treatment after percutaneous coronary intervention: a meta-analysis. Mol Biol Rep 41, 6263–6273 (2014). https://doi.org/10.1007/s11033-014-3509-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3509-7