Abstract
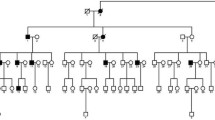
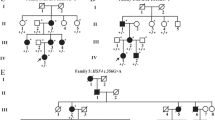
Cataract, defined as any opacity of the crystallin lens, can be divided into early onset (congenital or infantile) and age-related. It is the leading cause of visual disability in children, and mutations in many genes have currently been linked with this disorder. In the present study, we identified a genetic defect in a Chinese family with congenital cataract. Genomic DNA was extracted from the venous blood of the family and 100 normal controls. To screen for the disease-causing mutation, we sequenced eight candidate genes, and to predict the functional consequences of the mutation, a structural model of the protein was developed using the Protein Data Bank and PyMOL 1.1r1. We found a novel variant (c.163 A > G transition) in the gene for gap junction protein α3, or the connexin46 gene. This mutation resulted in the substitution of a highly conserved asparagine at codon 55 by aspartic acid (p.N55D). There were no nucleotide polymorphisms in the other candidate genes sequenced.




Similar content being viewed by others
References
Francis PJ, Berry V, Bhattacharya SS, Moore AT (2000) The genetics of childhood cataract. J Med Genet 37:481–488
Hejtmancik JF (2008) Congenital cataracts and their molecular genetics. Semin Cell Dev Biol 19:134–149
Litt M, Kramer P, LaMorticella DM, Murphey W, Lovrien EW, Weleber RG (1998) Autosomal dominant congenital cataract associated with a missense mutation in the human alpha crystallin gene CRYAA. Hum Mol Genet 7:471–474
Berry V, Francis P, Reddy MA, Collyer D, Vithana E, MacKay I, Dawson G, Carey AH, Moore A, Bhattacharya SS, Quinlan RA (2001) Alpha-B crystallin gene (CRYAB) mutation causes dominant congenital posterior polar cataract in humans. Am J Hum Genet 69:1141–1145
Qi Y, Jia H, Huang S, Lin H, Gu J, Su H, Zhang T, Gao Y, Qu L, Li D, Li Y (2004) A deletion mutation in the betaA1/A3 crystallin gene (CRYBA1/A3) is associated with autosomal dominant congenital nuclear cataract in a Chinese family. Hum Genet 114:192–197
Billingsley G, Santhiya ST, Paterson AD, Ogata K, Wodak S, Hosseini SM, Manisastry SM, Vijayallakshmi P, Gopinath PM, Graw J, Heon E (2006) CRYBA4 a novel human cataract gene is also involved in microphthalmia. Am J Hum Genet 79:702–709
Mackay DS, Boskovska OB, Knopf HLS, Lampi KJ, Shiels A (2002) A nonsense mutation in CRYBB1 associated with autosomal dominant cataract linked to human chromosome 22q. Am J Hum Genet 71:1216–1221
Litt M, Carrero-Valenzuela R, LaMorticella DM, Schultz DW, Mitchell TN, Kramer P, Maumenee IH (1997) Autosomal dominant cerulean cataract is associated with a chain termination mutation in the human beta-crystallin gene CRYBB2. Hum Mol Genet 6:665–668
Riazuddin SA, Yasmeen A, Yao W, Sergeev YV, Zhang Q, Zulfiqar F, Riaz A, Riazuddin S, Hejtmancik JF (2005) Mutations in beta-B3-crystallin associated with autosomal recessive cataract in two Pakistani families. Invest Ophthalmol Vis Sci 46:2100–2106
Héon E, Priston M, Schorderet DF, Billingsley GD, Girard PO, Lubsen N, Munier FL (1999) The gamma-crystallins and human cataracts: a puzzle made clearer. Am J Hum Genet 65:1261–1267
Stephan DA, Gillanders E, Vanderveen D, Freas-Lutz D, Wistow G, Baxevanis AD, Robbins CM, VanAuken A, Quesenberry MI, Bailey-Wilson J, Juo S-HH, Trent JM, Smith L, Brownstein MJ (1999) Progressive juvenile-onset punctuate cataracts caused by mutation of the gamma-D-crystallin gene. Proc Natl Acad Sci USA 96:1008–1012
Sun H, Ma Z, Li Y, Liu B, Li Z, Ding X, Gao Y, Ma W, Tang X, Li X, Shen Y (2005) Gamma-S crystallin gene (CRYGS) mutation causes dominant progressive cortical cataract in humans. J Med Genet 42:706–710
Shiels A, Mackay D, Ionides A, Berry V, Moore A, Bhattacharya S (1998) A missense mutation in the human connexin50 gene (GJA8) underlines autosomal dominant “zonular pulverulent” cataract, on chromosome 1q. Am J Hum Genet 62:526–532
Mackay D, Ionides A, Kibar Z, Rouleau G, Berry V, Moore A, Shiels A, Bhattacharya S (1999) Connexin46 mutations in autosomal dominant congenital cataract. Am J Hum Genet 64:1357–1364
Bu L, Jin Y, Shi Y, Chu R, Ban A, Eiberg H, Andres L, Jiang H, Zheng G, Qian M, Cui B, Xia Y, Liu J, Hu L, Zhao G, Hayden MR, Kong X (2002) Mutant DNA-binding domain of HSF4 is associated with autosomal dominant lamellar and Marner cataract. Nat Genet 31:276–278
Berry V, Francis P, Kaushal S, Moore A, Bhattacharya S (2000) Missense mutations in MIP underlie autosomal dominant ‘polymorphic’ and lamellar cataracts linked to 12q. Nat Genet 25:15–17
Conley YP, Erturk D, Keverline A, Mah TS, Keravala A, Barnes LR, Bruchis A, Hess JF, FitzGerald PG, Weeks DE, Ferrell RE, Gorin MB (2000) A juvenile-onset, progressive cataract locus on chromosome 3q21-q22 is associated with a missense mutation in the beaded filament structural protein-2. Am J Hum Genet 66:1426–1431
Ramachandran RD, Perumalsamy V, Hejtmancik JF (2007) Autosomal recessive juvenile onset cataract associated with mutation in BFSP1. Hum Genet 121:475–482
Pfenniger A, Wohlwend A, Kwak BR (2011) Mutations in connexin genes and disease. Eur J Clin Invest 41:103–116
Brady Trexler E, Feliksas F (2000) The first extracellular loop domain is a major determinant of charge selectivity in connexin46 channels. Biophys J 79(6):3036–3051
Willecke K, Jungbluth S, Dahl E, Hennemann H, Heynkes R, Grzeschik K-H (1990) Six genes of the human connexin gene family coding for gap junctional proteins are assigned to four different human chromosomes. Eur J Cell Biol 53:275–280
Kumar NM, Gilula NB (1996) The gap junction communication channel. Cell 84:381–388
Simon AM, Goodenough DA (1998) Diverse functions of vertebrate gap junctions. Trends Cell Biol 8:477–483
Jiang JX, Goodenough DA (1996) Heteromeric connexons in lens gap junction channels. Proc Natl Acad Sci USA 93:1287–1291
Verselis VK, Ginter CS, Bargiello TA (1994) Opposite voltage gating polarities of two closely related connexins. Nature 368:348–351
Yeager M, Nicholson BJ (1996) Structure of gap junction intercellular channels. Curr Opin Struct Biol 6:183–192
Trexler EB, Bukauskas FF, Kronengold J, Bargiello TA, Verselis VK (2000) The first extracellular loop domain is a major determinant of charge selectivity in connexin46 channels. Biophys J 79:3036–3051
Acknowledgments
The authors are grateful to all patients and their family as well as to the normal volunteers for their participation in this research. This study was supported by the National Natural Science Foundation of China (81070730, 81271000), the Key Program of Natural Science Foundation of Heilongjiang Province (ZD201015), and the Foundation of Health Department of Heilongjiang Province (2011-054).
Author information
Authors and Affiliations
Corresponding author
Additional information
Ying Hu and Lin Gao have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hu, Y., Gao, L., Feng, Y. et al. Identification of a novel mutation of the gene for gap junction protein α3 (GJA3) in a Chinese family with congenital cataract. Mol Biol Rep 41, 4753–4758 (2014). https://doi.org/10.1007/s11033-014-3346-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3346-8