Abstract
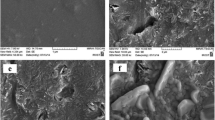
The aim of this work was to produce and characterize solid lipid nanoparticles (SLN) containing levothyroxine sodium for oral administration, and to evaluate the kinetic release of these colloidal carriers. SLNs were prepared by microemulsion method. The particle size and zeta potential of levothyroxine sodium-loaded SLNs were determined to be around 153 nm,−43 mV (negatively charged), respectively by photon correlation spectroscopy. The levothyroxine entrapment efficiency was over 98 %. Shape and surface morphology were determined by TEM and SEM. They revealed fairly spherical shape of nanoparticles.SLN formulation was stable over a period of 6 months. There were no significant changes in particle size, zeta potential and polydispersity index and entrapment efficiency, indicating that the developed SLNs were fairly stable.




Similar content being viewed by others
References
Alexander KS, Kothapalli MR, Dollimor D (1997) Stability of an extemporaneously formulated levothyroxine sodium syrup compounded from commercial tablets. Int J Pharm Compd 1(1):60–64
Ali H, El-Sayed K, Sylvester PW, Nazzal S (2010) Molecular interaction and localization of tocotrienol-rich fraction (TRF) within the matrices of lipid nanoparticles: evidence studies by differential scanning calorimetry (DSC) and proton nuclear magnetic resonance spectroscopy (1H NMR). Colloids Surf B 77(2):286–297
Almeida AJ, Souto E (2007) Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv Drug Deliv Rev 59(6):478–490
Arza RAK, Gonugunta CSR, Veerareddy PR (2009) Formulation and evaluation of swellable and floating gastroretentive ciprofloxacin hydrochloride tablets. Am Assoc Pharm Sci 1(10):220–226
Barzegar-Jalali M, Adibkia K, Valizadeh H, Siahi-Shadbad MR, Nokhodchi A, Omidi Y, Mohammadi G, Hallaj-Nezhadi S, Hasan M (2008) Kinetic analysis of drug release from nanoparticles. J Pharm Pharm Sci 11(1):167–177
Blakesley VA (2005) Current methodology to assess bioequivalence of levothyroxine sodium products is inadequate. J Am Assoc Pharm Sci 7(1):E42–E46
Cavalli R, Bargoni A, Podio V, Muntoni E, Zara GP, Gasco MR (2003) Duodenal administration of solid lipid nanoparticles loaded with different percentages of Tobramycin. J Pharm Sci 92(5):1085–1094
Chen DB, Yang TZ, Lu WL, Zhang Q (2001) In vitro and in vivo study of two types of long-circulating solid lipid nanoparticles containing paclitaxel. Chem Pharm Bull 49(11):1444–1447
Costa P, Sousa Lobo JM (2001) Modeling and comparison of dissolution profiles. Eur J Pharm Sci 13(2):123–133
Derakhshandeh K, Erfan M, Dadashzadeh S (2007) Encapsulation of 9-nitrocamptothecin, a novel anticancer drug, in biodegradable nanoparticles: Factorial design, characterization and release kinetics. Eur J Pharm Biopharm 66(1):34–41
Ekambaram P, Hasan-Sathali AA, Priyanka K (2012) Solid lipid nanoparticles: a review. Sci Rev Chem Commun 2(1):80–102
Elgart A, Cherniakov I, Aldouby Y, Domb AJ, Hoffman A (2012) Lipospheres and pro-nanolipospheres for delivery of poorly water soluble compounds. Chem Phys Lipids 165(4):438–453
Freitas C, Muller RH (1998) Effect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLN) dispersions. Int J Pharm 168(2):221–229
Garcia-Fuentes M, Torres D, Alonso MJ (2002) Design of lipid nanoparticles for the oral delivery of hydrophilic macromolecules. Colloids Surf B 27(2–3):159–168
Heiati H, Tawashi R, Phillips NC (1998) Solid lipid nanoparticles as drug carriers II. Plasma stability and biodistribution of solid lipid nanoparticles containing the lipophilic prodrug 3 %-azido-3 %-deoxythymidinepalmitate in mice. Int J Pharm 174(4):71–80
Heydenreich AV, Westmeier R, Pedersen N, Poulsen HS, Kristensen HG (2003) Preparation and purification of cationic solid lipid nanospheres-effects on particle size, physical stability and cell toxicity. Int J Pharm 254(1):83–87
Hu FQ, Jiang SP, Du YZ, Yuan H, Ye YQ, Zeng S (2005) Preparation and characterization of stearic acid nanostructured lipid carriers by solvent diffusion method in an aqueous system. Colloids Surf B 45(3–4):167–173
Kamble MS, Vaidya KK, Bhosale AV, Chaudhari PD (2002) Solid lipid nanoparticles and nanostructure lipid carriers—an over view. Int J Pharm Chem Biol Sci 2(4):681–691
Kashanian S, Hemati Azandaryani A, Derakhshandeh K (2011) New surface modified solid lipid nanoparticles by using N-glutarylphosphatidylethanolamine as outer shell. Int J Nanomed 6(1–9):1–9
Kaura IP, Bhandari R, Bhandari S, Kakkar V (2008) Potential of solid lipid nanoparticles in brain targeting. J Control Release 127(2):97–109
Lilja JJ, Laitinen K, Neuvonen PJ (2005) Effects of grapefruit juice on the absorption of levothyroxine. Br J Clin Pharmacol 60(3):337–341
Li XW, Lin XH, Zheng LQ, Yu L, Lv F, Zhang Q, Liu WC (2008) Effect of poly(ethylene glycol) stearate on the phase behavior of monocaprate/Tween80/water system and characterization of poly(ethylene glycol) stearate-modified solid lipid nanoparticles. Colloids Surf A 317(1–3):352–359
Liu J, Gong T, Wang C, Zhong Z, Zhang Z (2007) Solid lipid nanoparticles loaded with insulin by sodium cholate-phosphatidylcholine-based mixed micelles: preparation and characterization. Int J Pharm 340(1–2):153–162
Mehnert W, Mader K (2001) Solid lipid nanoparticles Production, characterization and applications. Adv Drug Deliv Rev 47(2-3):165–196
Muller RH, Mader K, Gohla K (2000) Solid lipid nanoparticles (SLN) for controlled drug delivery—a review of the state of the art. Eur J Pharm Biopharm 50(1):161–177
Parhi R, Suresh P (2010) Production of solid lipid nanoparticles-drug loading and release mechanism. J Chem Pharm Res 2(1):211–227
Pedersen N, Hansen S, Heydenreich AV, Kristensen HG, Poulsen HS (2006) Solid lipid nanoparticles can effectively bind DNA, streptavidin and biotinylated ligands. Eur J Pharm Biopharm 62(2):155–162
Ruktanonchai U, Bejrapha P, Sakulkhu U, Opanasopit P, Bunyapraphatsara N, Junyaprasert V, Puttipipatkhachorn S (2009) Physicochemical characteristics, cytotoxicity, and antioxidant activity of three lipid nanoparticulate formulations of alpha-lipoic acid. Am Assoc Pharm Sci 10(1):227–234
Sarmento B, Martins S, Ferreira D, Souto BE (2007) Oral insulin delivery by means of solid lipid nanoparticles. Int J Nanomed 2(4):743–749
Singhvi G, Singh M (2011) Review: in vitro drug release characterization. Int J Pharm Stud Res 2(1):77–84
Üner M, Yener G (2007) Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. Int J Nanomed 2(3):289–300
Varelas CG, Dixon DG, Steiner CA (1995) Zero-order release from biphasic polymer hydrogels. J Control Release 34(3):185–192
Yang S, Zhu J, Lu Y, Liang B, Yang C (1999) Body distribution of camptothecin solid lipid nanoparticles after oral administration. Pharm Res 16(5):751–757
Zara GP, Bargoni A, Cavalli R, Fundaro A, Vighetto D, Gasco MR (2002) Pharmacokinetics and tissue distribution of Idarubicin-loaded solid lipid nanoparticles after duodenal administration to rats. J Pharma Sci 91(5):1324–1333
Zhang Q, Yie G, Li Y, Yang Q, Nagai T (2000) Studies on the cyclosporin A loaded stearic acid nanoparticles. Int J Pharm 200(2):153–159
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rostami, E., Kashanian, S. & Azandaryani, A.H. Preparation of solid lipid nanoparticles as drug carriers for levothyroxine sodium with in vitro drug delivery kinetic characterization. Mol Biol Rep 41, 3521–3527 (2014). https://doi.org/10.1007/s11033-014-3216-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3216-4