Abstract
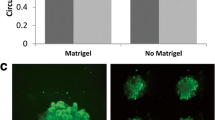
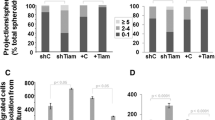
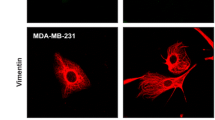
Interactions with stromal components influence the growth, survival, spread, and colonization capacities of tumor cells. Fibroblasts and macrophages which are responsible for the stroma production and maintenance are of the basic elements found in tumor microenvironment. Cellular density and ratio of stromal cells to tumor cells can also have modulatory effects in cancer. Here, the contribution of fibroblast and/or macrophage cells on the malignant behavior of breast cancer cells was modeled in co-culture systems. Co-cultures were established at different cell densities and ratios with 4T1 breast cancer, NIH/3T3 or 3T3-L1 fibroblast, and J774A.1 monocyte/macrophage cell lines. Flow cytometry-based proliferation, 3D growth on alginate matrix, and matrigel invasion assays were performed to determine the change in the malignant assets of tumor cells. The data were also supported by immunocytochemical and morphological analyses. Co-culturing with fibroblasts (especially, NIH/3T3 cells) significantly supported the proliferation, scattering, and invasiveness of 4T1 cells whereas inclusion of macrophages disrupted this positive influence. On the other hand, the invasion capacity of 4T1 cells was not enhanced in the co-cultures with fibroblasts whose motility were inhibited with pertussis toxin pretreatment. Particularly at low-density seeding in 3D cultures, 4T1 cells could form substantially more spheroids than that of in the co-cultures with fibroblasts. Only, increasing the amount of fibroblasts could restore the 3D-growth. Intriguingly, co-existence of macrophage, fibroblast, and tumor cells in 3D cultures provided a convenient stroma sustaining the spheroid formation and growth. In conclusion, fibroblasts can form a favorable environment for tumor cells’ spread and motility whereas restricting their 3D-growth capacity. On the other hand, presence of macrophages may disrupt the influence of fibroblasts and enhance the spheroid formation by the tumor cells.





Similar content being viewed by others
References
Mantovani A, Sica A, Allavena P, Garlanda C, Locati M (2009) Tumor-associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Hum Immunol 70:325–330
Kalluri R, Zeisberg M (2006) Fibroblasts in cancer. Nat Rev Cancer 6:392–401
Pietras A, Ostman A (2010) Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res 316:1324–1331
Arendt LM, Rudnick JA, Keller PJ, Kuperwasser C (2010) Stroma in breast development and disease. Semin Cell Dev Biol 21:11–18
Lu P, Weaver VM, Werb Z (2012) The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 196:395–406
Bissell MJ, Radisky D (2001) Putting tumours in context. Nat Rev Cancer 1:46–54
Olsen CJ, Moreira J, Lukanidin EM, Ambartsumian NS (2010) Human mammary fibroblasts stimulate invasion of breast cancer cells in a three-dimensional culture and increase stroma development in mouse xenografts. BMC Cancer 10:444–460
Casey T, Bond J, Tighe S, Hunter T, Lintault L, Patel O, Eneman J, Crocker A, White J, Tessitore J, Stanley M, Harlow S, Weaver D, Muss H, Plaut K (2009) Molecular signatures suggest a major role for stromal cells in development of invasive breast cancer. Breast Cancer Res Treat 114:47–62
Mendoza M, Khanna C (2009) Revisiting the seed and soil in cancer metastasis. Int J Biochem Cell Biol 41:1452–1462
Sadlonova A, Novak Z, Johnson MR, Bowe DB, Gault SR, Page GP, Thottassery JV, Welch DR, Frost AR (2005) Breast fibroblasts modulate epithelial cell proliferation in three-dimensional in vitro co-culture. Breast Cancer Res 7:R46–R59
Shimoda M, Mellody KT, Orimo A (2010) Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Semin Cell Dev Biol 21:19–25
Orimo A, Weinberg RA (2006) Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle 5:1597–1601
Radisky ES, Radisky DC (2007) Stromal induction of breast cancer: inflammation and invasion. Rev Endocr Metab Disord 8:279–287
Sugimoto H, Mundel TM, Kieran MW, Kalluri R (2006) Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther 5:1640–1646
Rama D, Esendagli G, Guc D (2011) Expression of chemokine-like receptor 1 (CMKLR1) on J744A.1 macrophages co-cultured with fibroblast and/or tumor cells: modeling the influence of microenvironment. Cell Immunol 271:134–140
Shapiro L, Cohen S (1997) Novel alginate sponges for cell culture and transplantation. Biomaterials 18:583–590
Fentiman I (1980) Cell communication in breast cancer. Ann R Coll Surg Engl 62:280–286
Casbas-Hernandez P, Fleming JM, Troester MA (2011) Gene expression analysis of in vitro cocultures to study interactions between breast epithelium and stroma. J Biomed Biotechnol 2011:520987
Place AE, Jin Huh S, Polyak K (2011) The microenvironment in breast cancer progression: biology and implications for treatment. Breast Cancer Res 13:227–238
Singer CF, Gschwantler-Kaulich D, Fink-Retter A, Haas C, Hudelist G, Czerwenka K, Kubista E (2008) Differential gene expression profile in breast cancer-derived stromal fibroblasts. Breast Cancer Res Treat 110:273–281
Goralski KB, Sinal CJ (2009) Elucidation of chemerin and chemokine-like receptor-1 function in adipocytes by adenoviral-mediated shRNA knockdown of gene expression. Methods Enzymol 460:289–312
Manabe Y, Toda S, Miyazaki K, Sugihara H (2003) Mature adipocytes, but not preadipocytes, promote the growth of breast carcinoma cells in collagen gel matrix culture through cancer-stromal cell interactions. J Pathol 201:221–228
Jacob S, Shastry P, Sudhakaran P (2002) Monocyte-macrophage differentiation in vitro: modulation by extracellular matrix protein substratum. Mol Cell Biochem 233:9–17
Pampaloni F, Reynaud EG, Stelzer EHK (2007) The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 8:839–845
Campbell JJ, Davidenko N, Caffarel MM, Cameron RE, Watson CJ (2011) A multifunctional 3D co-culture system for studies of mammary tissue morphogenesis and stem cell biology. PLoS One 6:e25661
Li L, Lu Y (2011) Optimizing a 3D culture system to study the interaction between epithelial breast cancer and its surrounding fibroblasts. J Cancer 2:458–466
Hughes CS, Postovit LM, Lajoie GA (2010) Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10:1886–1889
Taira M, Saitoh S, Sasaki K, Araki Y (2005) Three-dimensional culture of L929 and UMR106 cells in self-prepared alginate sponges. J Oral Tissue Eng 2:86–91
Stephan S, Johnson WE, Roberts S (2011) The influence of nutrient supply and cell density on the growth and survival of intervertebral disc cells in 3D culture. Eur Cell Mater 22:97–108
Silzle T, Kreutz M, Dobler MA, Cameron RE, Watson CJ (2003) Tumor-associated fibroblasts recruit blood monocytes into tumor tissue. Eur J Immunol 33:1311–1320
Acknowledgments
This study was supported by Hacettepe University Scientific Research Unit.
Conflict of interest
The authors declare no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rama-Esendagli, D., Esendagli, G., Yilmaz, G. et al. Spheroid formation and invasion capacity are differentially influenced by co-cultures of fibroblast and macrophage cells in breast cancer. Mol Biol Rep 41, 2885–2892 (2014). https://doi.org/10.1007/s11033-014-3144-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3144-3