Abstract
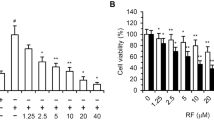
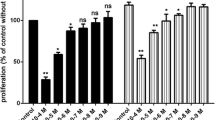
In the present investigation a novel series of chalcone analogues were synthesized and evaluated for their anti-proliferative activity in human umbilical vein endothelial cells (HUVECs). Among 14 tested compounds, chalcone analogue (E)-3-(2′-methoxybenzylidene)-4-chromanone (KRP6) exhibited the most potent activity with IC50 19 μM. Moreover, HUVECs exhibited divergent, even opposing concentration-dependent responses to KRP6. This compound was the most potent inhibitor of cell proliferation and extracellular matrix formation (fibronectin and type IV collagen) at higher concentrations (20–50 μM). In contrast, KRP6 stimulated the compensatory increase in proliferative activity including extracellular matrix formation at low concentrations (1, 10 μM). KRP6 concentration-dependently modulated phosphorylation of Akt and mitogen-activated protein kinases such as extracellular signal-regulated kinase-1/-2 and p38 kinase, suggesting that these pathways play a role in the effect mediated by this compound. In addition, we found a selective effect on activated endothelial cells, in particular with resting endothelial cells. In conclusion, KRP6 is a potent modulator of selected steps of the angiogenic process in vitro. Accordingly, further in vivo research should be performed to facilitate its use in clinical practice.




Similar content being viewed by others
References
Senevirathne M, Kim SK (2012) Utilization of seafood processing by-products: medicinal applications. Adv Food Nutr Res 65:495–512
Costa G, Francisco V, Lopes MC, Cruz MT, Batista MT (2012) Intracellular signaling pathways modulated by phenolic compounds: application for new anti-inflammatory drugs discovery. Curr Med Chem 19:2876–2900
Tsubura A, Lai YC, Kuwata M, Uehara N, Yoshizawa K (2011) Anticancer effects of garlic and garlic-derived compounds for breast cancer control. Anticancer Agents Med Chem 11:249–253
Aggarwal BB, Van Kuiken M, Iye LH, Harikumar KB, Sung B (2009) Molecular targets of nutraceuticals derived from dietary spices: potential role in suppression of inflammation and tumorigenesis. Exp Biol Med 234:825–849
Kim YH, Shin EK, Kim DH, Lee HH, Park JHY, Kim JK (2010) Antiangiogenic effect of licochalcone. A Biochem Pharmacol 80:1152–1159
Dyrager C, Wickström M, Fridén-Saxin M, Friberg A, Dahlén K, Wallén EA, Gullbo J, Grøtli M, Luthman K (2011) Inhibitors and promoters of tubulin polymerization: synthesis and biological evaluation of chalcones and related dienones as potential anticancer agent. Bioorg Med Chem 19:2659–2665
Batovska DI, Todorova IT (2010) Trends in utilization of the pharmacological potential of chalcones. Curr Clin Pharmacol 5:1–29
Yadav VR, Prasad S, Sung B, Aggarwal BB (2011) The role of chalcones in suppression of NF-κB-mediated inflammation and cancer. Int Immunopharmacol 11:295–309
Hseu YC, Lee MS, Wu CR, Cho HJ, Lin KY, Lai GH, Wang SY, Kuo YH, Kumar KJ, Yang HL (2012) The chalcone flavokawain B induces G2/M cell-cycle arrest and apoptosis in human oral carcinoma HSC-3 cells through the intracellular ROS generation and downregulation of the Akt/p38 MAPK signaling pathway. J Agric Food Chem 60:2385–2397
Kamal A, Mallareddy A, Suresh P, Shaik TB, Lakshma Nayak V, Kishor C, Shetti RV, Sankara Rao N, Tamboli JR, Ramakrishna S, Addlagatta A (2012) Synthesis of chalcone-amidobenzothiazole conjugates as antimitotic and apoptotic inducing agents. Bioorg Med Chem 20:3480–3492
Pilatova M, Varinska L, Perjesi P, Sarissky M, Mirossay L, Solar P, Ostro A, Mojzis J (2010) In vitro antiproliferative and antiangiogenic effects of synthetic chalcone analogues. Toxicol In Vitro 24:1347–1355
Xiao XY, Hao M, Yang XY, Ba Q, Li M, Ni SJ, Wang LS, Du X (2011) Licochalcone A inhibits growth of gastric cancer cells by arresting cell cycle progression and inducing apoptosis. Cancer Lett 302:69–75
Jing H, Zhou X, Dong X, Cao J, Zhu H, Lou J, Hu Y, He Q, Yang B (2010) Abrogation of Akt signaling by isobavachalcone contributes to its anti-proliferative effects towards human cancer cells. Cancer Lett 294:167–177
Rajendran P, Ong TH, Chen L, Li F, Shanmugam MK, Vali S, Abbasi T, Kapoor S, Sharma A, Kumar AP, Hui KM, Sethi G (2011) Suppression of signal transducer and activator of transcription 3 activation by butein inhibits growth of human hepatocellular carcinoma in vivo. Clin Cancer Res 17:1425–1439
Mojzis J, Varinska L, Mojzisova G, Kostova I, Mirossay L (2008) Antiangiogenic effect of flavonoids and chalcones. Pharmacol Res 57:259–265
Orlikova B, Tasdemir D, Golais F, Dicato M, Diederich M (2011) Dietary chalcones with chemopreventive and chemotherapeutic potential. Genes Nutr 6:125–147
Kong Y, Wang K, Edler MC, Hamel E, Mooberry SL, Paige MA, Brown ML (2010) A boronic acid chalcone analog of combretastatin A-4 as a potent anti-proliferation agent. Bioorg Med Chem 18:971–977
Robinson TP, Hubbard RB, Ehlers TJ, Arbiser JL, Goldsmith DJ, Bowen JP (2005) Synthesis and biological evaluation of aromatic enones related to curcumin. Bioorg Med Chem 13:4007–4013
Lee JS, Kang Y, Kim JT, Thapa D, Lee ES, Kim JA (2012) The anti-angiogenic and anti-tumor activity of synthetic phenyl propenone derivatives is mediated through the inhibition of receptor tyrosine kinases. Eur J Pharmacol 677:22–30
Noonan DM, Benelli R, Albini A (2007) Angiogenesis and cancer prevention: a vision. Recent Results Cancer Res 174:219–224
Park SY, Ku SK, Lee ES, Kim JA (2012) 1,3-Diphenylpropenone ameliorates TNBS-induced rat colitis through suppression of NF-κB activation and IL-8 induction. Chem Biol Interact 196:39–49
van Hinsbergh VW, Sprengers ED, Kooistra EA (1987) Effect of thrombin on the production of plasminogen activators and PA inhibitor-1 by human foreskin microvascular endothelial cells. Thromb Haemost 57:148–153
Defilippi P, van Hinsbergh VWM, Bertolotto A, Rossino P, Silengo L, Tarone G (1991) Differential distribution and modulation of expression of alpha1/beta1 integrin on human endothelial cells. J Cell Biol 114:855–863
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application for proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Klima J, Lacina L, Dvorankova B, Herrmann D, Carnwath JW, Niemann H, Kaltner H, Andre S, Motlik J, Gabius HJ, Smetana K Jr (2009) Differential regulation of galectin expression/reactivity during wound healing in porcine skin and in cultures of epidermal cells with functional impact on migration. Physiol Res 58:873–884
Martínez-Poveda B, Quesada AR, Medina MA (2005) Hypericin in the dark inhibits key steps of angiogenesis in vitro. Eur J Pharmacol 516:97–103
Grant DS, Tashiro KI, Segui-Real B, Yamada Y, Martin GR, Kleinman HK (1989) Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro Cell 58:933–943
Vailhé B, Vittet D, Feige JJ (2011) In vitro models of vasculogenesis and angiogenesis. Lab Invest 81:439–452
Santarpia L, Lippman SM, El-Naggar AK (2012) Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets 16:103–119
Zhang X, Yeung ED, Wang J, Panzhinskiy EE, Tong C, Li W, Li J (2010) Isoliquiritigenin, a natural anti-oxidant, selectively inhibits the proliferation of prostate cancer cells. Clin Exp Pharmacol Physiol 37:841–847
Long X, Fan M, Bigsby RM, Nephew KP (2008) Apigenin inhibits antiestrogen-resistant breast cancer cell growth through estrogen receptor-alpha-dependent and estrogen receptor-alpha-independent mechanisms. Mol Cancer Ther 7:2096–2108
ElAttar TM, Virji AS (1999) Modulating effect of resveratrol and quercetin on oral cancer cell growth and proliferation. Anticancer Drugs 10:187–193
Baron-Menguy C, Bocquet A, Guihot AL, Chappard D, Amiot MJ, Andriantsitohaina R, Loufrani L, Henrion D (2007) Effects of red wine polyphenols on postischemic neovascularization model in rats: low doses are proangiogenic, high doses anti-angiogenic. FASEB J 21:3511–3521
Choi EJ, Kim GH (2013) Antiproliferative activity of daidzein and genistein may be related to ERα/c-erbB-2 expression in human breast cancer cells. Mol Med Rep. doi:10.3892/mmr.2013.1283
Panchapakesan G, Dhayalan V, Dhatchana Moorthy N, Saranya N, Mohanakrishnan AK (2011) Synthesis of 2-substituted 17β-hydroxy/17-methylene estratrienes and their in vitro cytotoxicity in human cancer cell cultures. Steroids 76:1491–1504
Solomon VR, Lee H (2011) Anti-breast cancer activity of heteroaryl chalcone derivatives. Biomed Pharmacother 66:213–220
Varinska L, van Wijhe M, Belleri M, Mitola S, Perjesi P, Presta M, Koolwijk P, Ivanova L, Mojzis J (2012) Anti-angiogenic activity of the flavonoid precursor 4-hydroxychalcone. Eur J Pharmacol 691:125–133
Acknowledgments
This work was supported by the Slovak Research and Development Agency under contract No. APVV-0325-07, by SEPO-II (ITMS code: 26220120039) and by Charles University (Grant No. PRVOUK 27-1). We would like to thank Tom Billingham for his careful proof-reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
L. Ivanova and L. Varinska contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ivanova, L., Varinska, L., Pilatova, M. et al. Cyclic chalcone analogue KRP6 as a potent modulator of cell proliferation: an in vitro study in HUVECs. Mol Biol Rep 40, 4571–4580 (2013). https://doi.org/10.1007/s11033-013-2547-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-013-2547-x